The mechanism of action of pasireotide and its clinical application in the management of Cushing’s disease will be reviewed.
Endocrine Physiology
The Hypothalamic-Pituitary-Adrenal Axis : The hypothalamic-Pituitary-Adrenal (HPA) axis influences cortisol regulation through a complex balancing act between stimulatory and inhibitory factors. Corticotrophin-releasing hormone (CRH), produced in the paraventricular nucleus of the hypothalamus, is ferried by the hypophyseal-portal venous system to corticotroph cells in the anterior pituitary gland(1). CRH subsequently binds Corticotrophin releasing hormone receptor type 1 (CRH-R1) receptors on the surface of corticotrophs. As a result, there is a release of ACTH from secretory vesicles in corticotrophs. It is worthy to note that Arginine vasopressin (AVP) further potentiates the anterior pituitary effects of CRH by acting on its cognate V1b receptor (V1b-R) present on corticotrophs. Also, CRH promotes pro-opiomelanocortin (POMC) gene expression in the anterior pituitary gland, a process that increases adrenocorticotropin hormone (ACTH) production as well (2).
Afterward, ACTH binds to the melanocortin-2 receptor (MCR-2) on cells present in the zona fasciculata of the adrenal cortex, which leads to increased cortisol synthesis(3). Adrenal-derived cortisol inhibits POMC and ACTH secretion by anterior pituitary corticotrophs through a negative feedback loop(4). Additional negative feedback inhibition of CRH and AVP synthesis by cortisol occurs at the level of the hypothalamic paraventricular nucleus(5)(see figure 1.0).
Normal and adenomatous corticotrophs express two subclasses of somatostatin receptors(SSRs), namely somatostatin receptor subtype 2 (SSR2) and somatostatin receptor subtype 5 (SSR5). Somatostatin, a hypothalamic peptide, inhibits ACTH production through an inhibitory pathway regulated by circulating cortisol. Indeed, SSR2 receptors are easily downregulated by cortisol, compared to the SSR5 (more resistant to negative feedback inhibition by cortisol). As a consequence, SSR2 receptor modulators (e.g., octreotide) are less effective in Cushing’s disease compared to SSR5 modulators (e.g., pasireotide)(6).
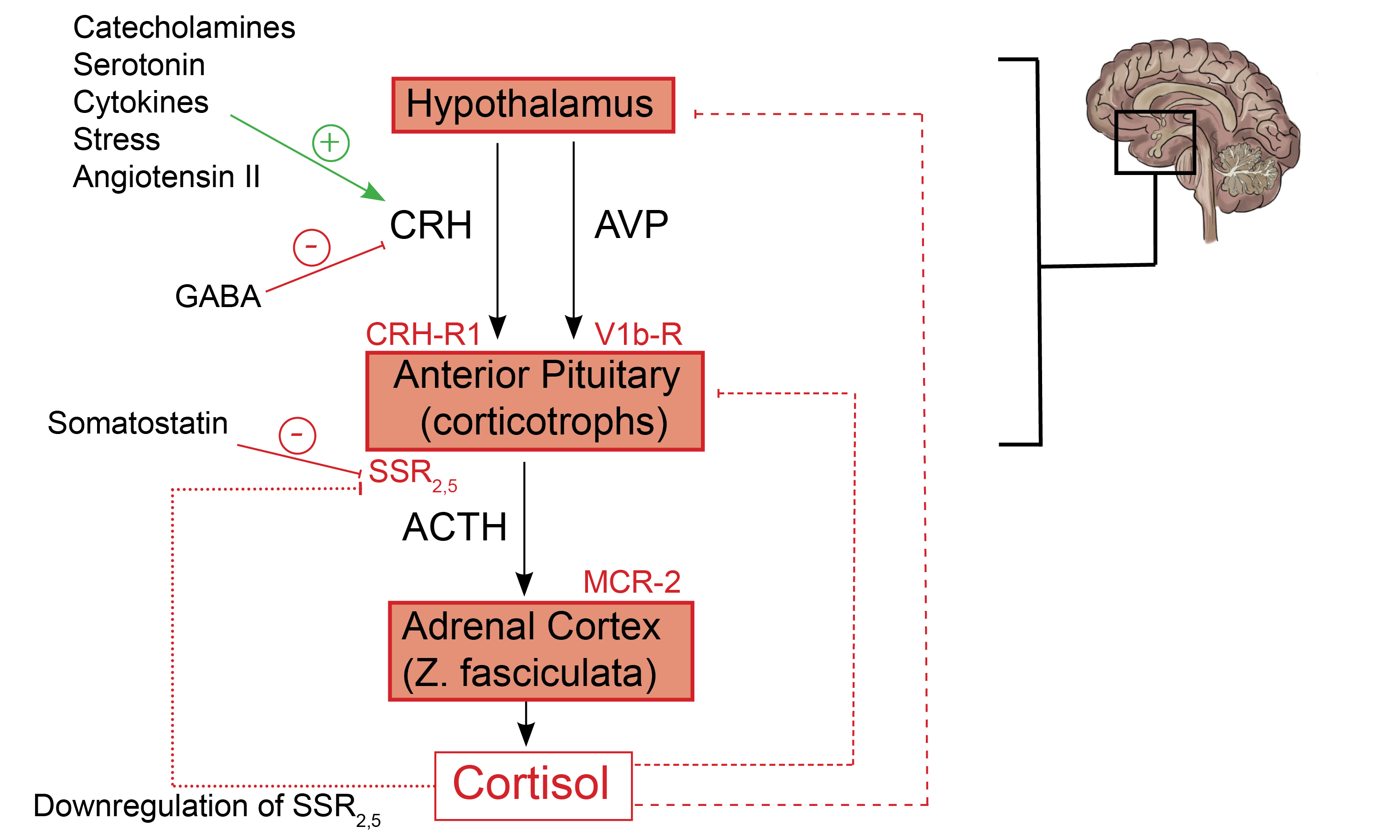
Fig. 1.0 Schematic representation of the HPA axis, highlighting critical stimulatory and inhibitory feedback loops. Hypothalamic and Pituitary processes: CRH is released under trophic stimulation by various factors, including catecholamines, Angiotensin II, serotonin, stress, and cytokines(7). In contrast, GABA inhibits CRH release and, ultimately, the production of ACTH(8). CRH from the hypothalamus stimulates anterior pituitary corticotrophs to release their pre-formed ACTH from secretory vesicles (fast response). Furthermore, CRH increases POMC gene expression by anterior pituitary corticotrophs (slow response).
Additionally, AVP from the PVN binds to V1b receptors on corticotrophs which further augments the action of CRH at the level of the anterior pituitary gland(2). Activation of dopamine D2 receptors (D2Rs) present on corticotroph cells by hypothalamic-derived dopamine inhibits ACTH synthesis and release (not shown) (9). Adrenal cortex processes: The binding of ACTH to MCR-2 receptors present on cells in the zona fasciculata promotes the synthesis of cortisol from cholesterol(3). Feedback inhibitory process: Negative feedback inhibition of POMC and ACTH release is mediated by adrenal-derived cortisol (short dashed line)(4). Also, cortisol inhibits the synthesis of CRH and AVP from paraventricular nuclei in the hypothalamus (long dashed line)(5). Cortisol-mediated inhibition of somatostatin receptor expression on corticotrophs affects SSR2 more than it does SSR5 (dotted line)(6). + = shows stimulatory factors and feedback loops, – = shows inhibitory factors and feedback loops. (Redrawn and modified from Spencer RL et al. (2017) A users guide to HPA axis research. Physiol Behav 178:43–65)
Mechanism of action
Pasireotide is a “near pan-somatostatin” receptor analog because it binds to four of the five somatostatin receptor isoforms (SSR1, SSR2, SSR3, and SSR5). Indeed, pasireotide binds to the SSR5 receptor subtype more avidly than the other somatostatin receptors, thus its demonstrable efficacy in Cushing’s disease. Corticotroph tumors in the anterior pituitary gland express more SSR5 receptors than the other somatostatin receptor subtypes. Moreover, cortisol’s negative feedback inhibition of somatostatin receptor expression by corticotrophs tends to impact SSR2 receptors more than it does the SSR5 receptor subtype. Due to its affinity for SSR5 receptors, pasireotide is an ideal therapeutic option in Cushing’s disease(10). Also, see figure 1.0.
Practice Guide
Pasireotide (Signifor) causes hyperglycemia, gastrointestinal discomfort, and cholelithiasis. The reported prevalence of hyperglycemia in clinical trials involving patients with Cushing’s disease who received pasireotide ranged from 68.4 to 73%(11). Hence, it is reasonable to screen for diabetes before and during treatment with pasireotide(12). Incretin mimetics, metformin, or insulin are preferred for treating pasireotide-mediated hyperglycemia(13).
Clinical Trial Evidence
SSR5 receptors are abundant on corticotroph tumors, as has been previously mentioned. The Pasireotide B2305 Study group trial (infographic) investigated the efficacy of pasireotide, a SSA with a profound affinity for the SSR5 receptor, in reducing corticotroph tumor growth(14).
Key Message
Pasireotide led to a halving of median urinary-free cortisol levels in a cohort of patients with confirmed Cushing’s disease (persistent, recurrent, or newly diagnosed).
The pasireotide B2305 study group evaluated the efficacy of pasireotide in Cushing’s disease. In this pivotal phase 3 trial, 162 subjects with persistent, recurrent, or newly diagnosed (not deemed suitable candidates for transsphenoidal surgery) Cushing’s disease with urinary free cortisol levels 1.5 times the upper limit of the normal reference range were randomized to subcutaneous pasireotide 600mcg (n=82) or 900mcg (n=80), twice daily. The primary outcome was defined as urinary-free cortisol (UFC) levels below or at the upper limit of the normal reference range. There was approximately a 50% reduction in median urinary-free cortisol levels by the second month of the study. UFC levels stabilized through to the end of the study for all participants(14).
References
- Martin-Grace J, Dineen R, Sherlock M, Thompson CJ. Adrenal insufficiency: Physiology, clinical presentation and diagnostic challenges. Clin Chim Acta Int J Clin Chem. 2020 Jun;505:78–91.
- Rotondo F, Butz H, Syro LV, Yousef GM, Di Ieva A, Restrepo LM, et al. Arginine vasopressin (AVP): a review of its historical perspectives, current research and multifunctional role in the hypothalamo-hypophysial system. Pituitary. 2016 Aug;19(4):345–55.
- Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2017 Sep 1;178:43–65.
- Osterlund CD, Rodriguez-Santiago M, Woodruff ER, Newsom RJ, Chadayammuri AP, Spencer RL. Glucocorticoid Fast Feedback Inhibition of Stress-Induced ACTH Secretion in the Male Rat: Rate Independence and Stress-State Resistance. Endocrinology. 2016 Jul;157(7):2785–98.
- Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 Jul 1;32(5):683–94.
- Hofland LJ, Lamberts SWJ, Feelders RA. Role of somatostatin receptors in normal and tumoral pituitary corticotropic cells. Neuroendocrinology. 2010;92 Suppl 1:11–6.
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016 Mar 15;6(2):603–21.
- Giordano R, Pellegrino M, Picu A, Bonelli L, Balbo M, Berardelli R, et al. Neuroregulation of the hypothalamus-pituitary-adrenal (HPA) axis in humans: effects of GABA-, mineralocorticoid-, and GH-Secretagogue-receptor modulation. ScientificWorldJournal. 2006 Jan 17;6:1–11.
- Tateno T, Kato M, Tani Y, Oyama K, Yamada S, Hirata Y. Differential expression of somatostatin and dopamine receptor subtype genes in adrenocorticotropin (ACTH)-secreting pituitary tumors and silent corticotroph adenomas. Endocr J. 2009;56(4):579–84.
- Lacroix A, Gu F, Schopohl J, Kandra A, Pedroncelli AM, Jin L, et al. Pasireotide treatment significantly reduces tumor volume in patients with Cushing’s disease: results from a Phase 3 study. Pituitary. 2020;23(3):203–11.
- Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19(5):536–43.
- Novartis Pharmaceuticals. A Randomized, Double-blind Study to Assess the Safety and Efficacy of Different Dose Levels of Pasireotide (SOM230) Subcutaneous (sc) Over a 6 Month Treatment Period in Patients With de Novo, Persistent or Recurrent Cushing’s Disease [Internet]. clinicaltrials.gov; 2016 Feb [cited 2020 May 22]. Report No.: NCT00434148. Available from: https://clinicaltrials.gov/ct2/show/NCT00434148
- Tritos NA, Biller BMK. Advances in the Medical Treatment of Cushing Disease. Endocrinol Metab Clin North Am. 2020 Sep 1;49(3):401–12.
- Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012 Mar 8;366(10):914–24.
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
Kindly Let Us Know If This Was helpful? Thank You!


