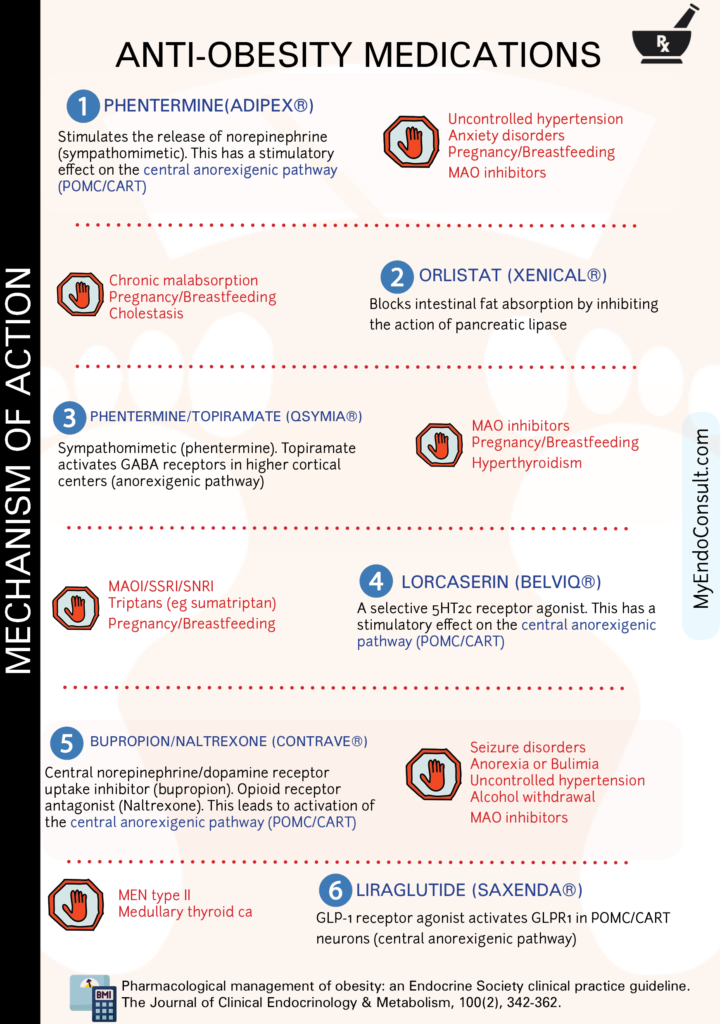
There are a number of medical treatments for obesity that can help people to lose weight and improve their health. One of the most common medications used for this purpose is phentermine, which works by reducing appetite and increasing energy levels. Another option is GLP-1 agonists, which help to regulate metabolism and appetite.
Other medications include orlistat, phentermine/topiramate, lorcaserin (recently withdrawn from the market), and bupropion/naltrexone. All of these medications can be effective in helping people to lose weight, but they should be used in conjunction with lifestyle changes such as diet and exercise.
Physiology of Appetite Regulation
The anorexigenic (satiety-promoting) and orexigenic (hunger-stimulating) pathways consist of an intricate network of hormones, neurotransmitters, and neural circuits, all intricately interwoven to maintain our body’s energy balance
The anorexigenic pathway is a complex signaling system that regulates food intake and energy balance. It involves communication between peripheral tissues, such as adipose tissue, and central nervous system structures, including the hypothalamus and higher cortical centers. The role of other mediators of the anorexigenic pathway is shown in Figure 1.0
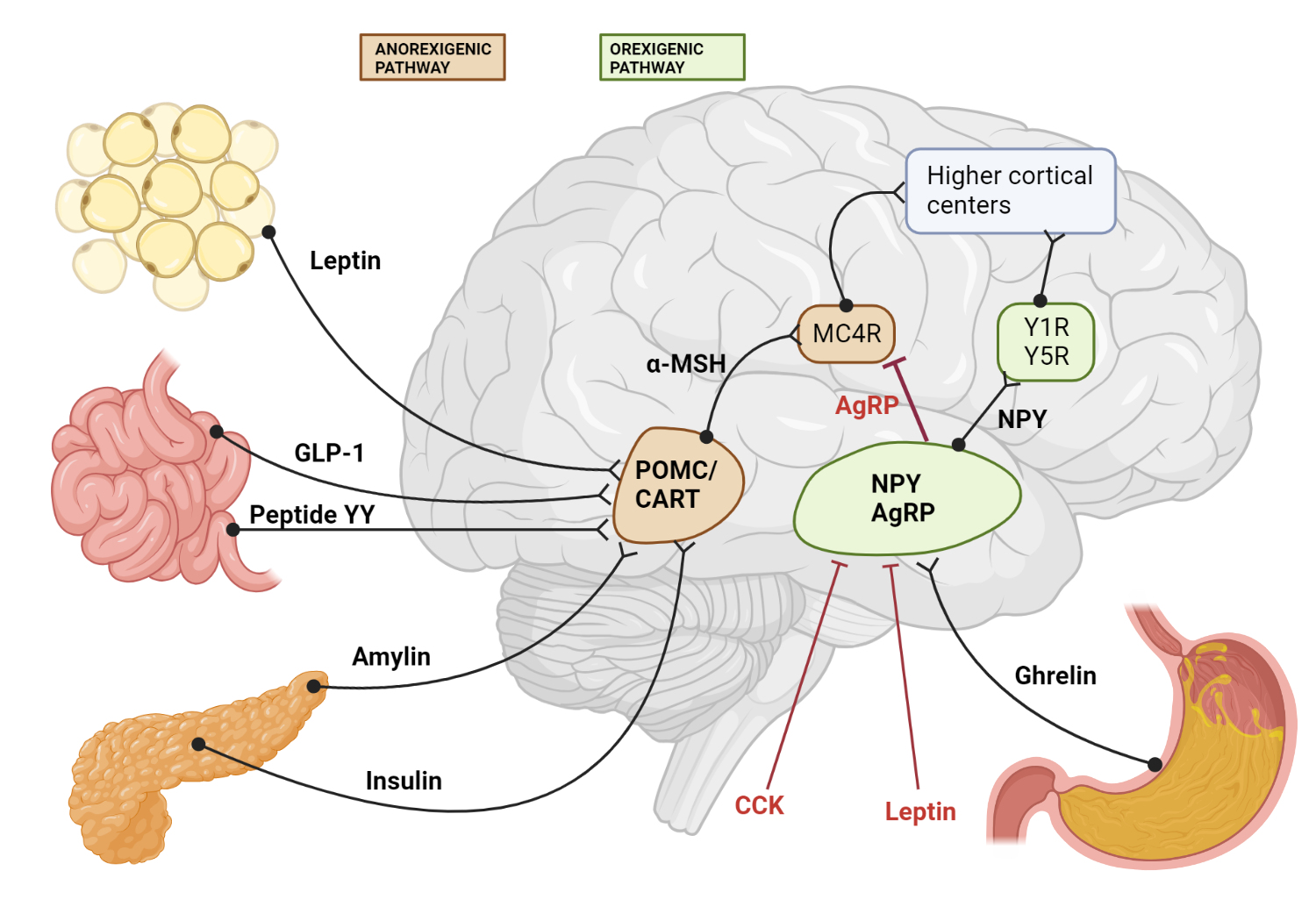
Figure 1.0 Schematic diagram of both orexigenic and anorexigenic pathways. There are connections between peripheral organs and relay centers in the hypothalamus en route to higher cortical centers.
Adipose tissue produces and releases the hormone leptin in proportion to the amount of stored fat. As fat stores increase, leptin levels rise, signaling the body to decrease food intake and increase energy expenditure.
Leptin circulates in the bloodstream, crosses the blood-brain barrier via specific transporters, and finally reaches the arcuate nucleus of the hypothalamus in order to exert its anorexigenic pathway.
The arcuate nucleus contains two distinct populations of neurons: one that produces neuropeptide Y (NPY) and agouti-related peptide (AgRP) and another that produces pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). Leptin binds to its receptors on these neurons, inhibiting NPY/AgRP neurons and stimulating POMC/CART neurons.
NPY is an orexigenic peptide, while AgRP acts as an antagonist of the melanocortin receptors. Leptin suppresses the orexigenic effects of NPY while promoting the anorexigenic pathway via POMC/CART activation, decreasing food intake and promoting weight loss.
POMC is processed into alpha-melanocyte-stimulating hormone (α-MSH), which binds to melanocortin receptors and exerts anorexigenic effects. CART also has anorexigenic properties. Activation of POMC/CART neurons by leptin contributes to reduced food intake and increased energy expenditure.
Signals from the arcuate nucleus are transmitted to higher cortical centers involved in the regulation of appetite, satiety, and energy balance[101]. These centers integrate information from various sources, including hormonal and neural signals, to modulate feeding behavior.
Phentermine
Phentermine is a sympathomimetic amine that acts as an appetite suppressant and is used for the short-term management of obesity in conjunction with a reduced-calorie diet and exercise. The precise mechanism of action of phentermine is not entirely understood, but it is believed to exert its effects primarily through the central nervous system by stimulating the release of certain neurotransmitters.
Phentermine stimulates the release of catecholamines, primarily norepinephrine (NE), and to a lesser extent, dopamine (DA) and serotonin (5-HT), in the hypothalamus and other regions of the brain.
The increased levels of norepinephrine, dopamine, and serotonin in the hypothalamus, specifically in the arcuate nucleus, lead to the activation of neurons that express pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). Activation of POMC/CART neurons promotes the release of alpha-melanocyte-stimulating hormone (α-MSH), which in turn binds to melanocortin receptors (MC4R) in the brain. This binding suppresses appetite and reduces food intake.
The starting dosage for phentermine-topiramate consists of 3.75/23 mg administered for a 14-day period, followed by a dose of 7.5/46 mg. If a 3% reduction in baseline body weight is not observed after 12 weeks, the dosage can be escalated to 11.25/69 mg for an additional 14 days and subsequently increased to 15/92 mg daily.
Orlistat
Orlistat is a gastrointestinal lipase inhibitor that is used to treat obesity. Its mechanism of action involves the reversible inhibition of pancreatic lipases, which are essential enzymes responsible for the hydrolysis of dietary triglycerides into absorbable free fatty acids and monoglycerides. By inhibiting these lipases, orlistat prevents the breakdown and subsequent absorption of dietary fat in the gastrointestinal tract.
Consequently, a portion of the consumed fat is excreted in the feces, reducing the overall caloric intake and promoting weight loss. It is important to note that orlistat does not act centrally; its effects are confined to the gastrointestinal tract.
Topiramate
Topiramate works by affecting GABA receptors in the brain. This action leads to an increase in satiety, thus reducing caloric intake.
Bupropion/Naltrexone
The combination of sustained-release (SR) bupropion and naltrexone has been shown to be effective in the treatment of obesity through their synergistic effects on appetite regulation and energy expenditure.
Bupropion, an atypical antidepressant, and a dopamine/norepinephrine reuptake inhibitor, is believed to promote weight loss through its stimulatory effect on pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. Activation of POMC neurons leads to the release of alpha-melanocyte-stimulating hormone (α-MSH), which binds to melanocortin-4 receptors (MC4R) in downstream brain regions. This binding results in a decrease in food intake and an increase in energy expenditure.
Naltrexone, an opioid receptor antagonist, primarily blocks the mu-opioid receptor. Its role in weight loss is thought to be related to its ability to counteract the inhibitory effect of beta-endorphins on POMC neurons. Under normal circumstances, beta-endorphins, which are opioid peptides, suppress POMC neuronal activity. By antagonizing the mu-opioid receptor, naltrexone prevents beta-endorphins from inhibiting POMC neurons, thus promoting the release of α-MSH and enhancing the anorexigenic pathway.
Initially, administer a single tablet containing a combination of 8 mg naltrexone and 90 mg bupropion once daily in the morning for a duration of one week. Subsequently, based on patient tolerability, escalate the dosage in weekly intervals as follows: during the second week, administer one tablet twice daily; during the third week, administer two tablets in the morning and one tablet in the evening; and thereafter, administer two tablets twice daily. The maximum recommended dosage is four tablets per day, equivalent to 32 mg naltrexone and 360 mg bupropion.
Lorcaserin
Lorcaserin (Belviq) is an anorexigenic pathway regulating drug that acts as an agonist of the serotonin receptor 5HT2C. The activation of this particular subtype of serotonin receptor is known to cause anorexia, satiety, and weight loss. Animal studies have shown that lorcaserin can reduce food intake and body weight by 10-15% after acute administration In humans, lorcaserin has been found to be an effective obesity treatment. In a 1-year trial, patients who were administered lorcaserin lost an average of 5% of their body weight, while those in the placebo group only lost an average of 2%
Medication update: The FDA has withdrawn lorcaserin, a medication used to treat obesity, due to concerns about its potential side effects. Lorcaserin was approved by the FDA in 2012 for use as an adjunct to a diet and exercise program for the treatment of obesity. However, recent studies have linked lorcaserin to an increased risk of cancer, so the FDA has decided to withdraw the drug from the market.
liraglutide
Liraglutide (Saxenda) is a medication used to treat obesity. It belongs to a class of medications known as GLP-1 receptor agonists. Liraglutide works by mimicking the effects of a hormone called GLP-1, which is released in response to food intake.
GLP-1 agonists stimulate the anorexigenic pathway by binding to GLP-1 receptors (GLP-1R) present on POMC/CART neurons in the arcuate nucleus of the hypothalamus. Upon activation by GLP-1, POMC/CART neurons stimulate the synthesis and release of alpha-melanocyte-stimulating hormone (α-MSH). Next, α-MSH binds to melanocortin-4 receptors (MC4R) found on second-order neurons in the hypothalamus, which relays signals to higher cortical centers that ultimately lead to early satiety.
For weight loss, liraglutide (Saxenda®) should be started at a dose of 0.6 mg subcutaneously once daily for one week. After the initial week, the dose should be increased by 0.6 mg increments each week until the maintenance dose of 3.0 mg once daily is reached. The dose should not exceed 3.0 mg daily, and liraglutide can be administered at any time of day, with or without meals.
For patients on semaglutide (Wegovy™), the initial dose should be 0.25 mg subcutaneously once weekly for four weeks. Following this period, the dose should be increased to 0.5 mg once weekly for the next four weeks. If further dose escalation is needed, the dose can be increased to 1.0 mg once weekly for four weeks. The dose can be increased to 2.0mg for a further month and then a maximum of 2.4mg weekly.
Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, which has shown promise in treating obesity. Tirzepatide’s site of action in the arcuate nucleus involves binding to GLP-1 receptors on POMC/CART neurons(anorexigenic pathway).
 Clinical Pearls
Clinical Pearls
How do corticosteroids promote weight gain?
As you may recall, alpha MSH is the ligand for the hypothalamic MC4 receptor (MC4R) in the anorexigenic pathway. Proopiomelanocortin in the hypothalamus is converted to ACTH, Beta-lipoprotein, and gamma melanocyte-stimulating hormone (γMSH). Next, γMSH is processed into alpha MSH (αMSH) and Corticotropin-like intermediate peptide (CLIP). Cortisol exerts negative feedback control POMC processing at the level of the hypothalamus under normal conditions. For patients on exogenous glucocorticoid therapy, there is profound suppression of POMC production and hence, αMSH production. This reduces signaling of the anorexigenic pathway in favor of the orexigenic pathway ( neuropeptide Y/agouti-related peptide). Review both the anorexigenic and orexigenic pathways.
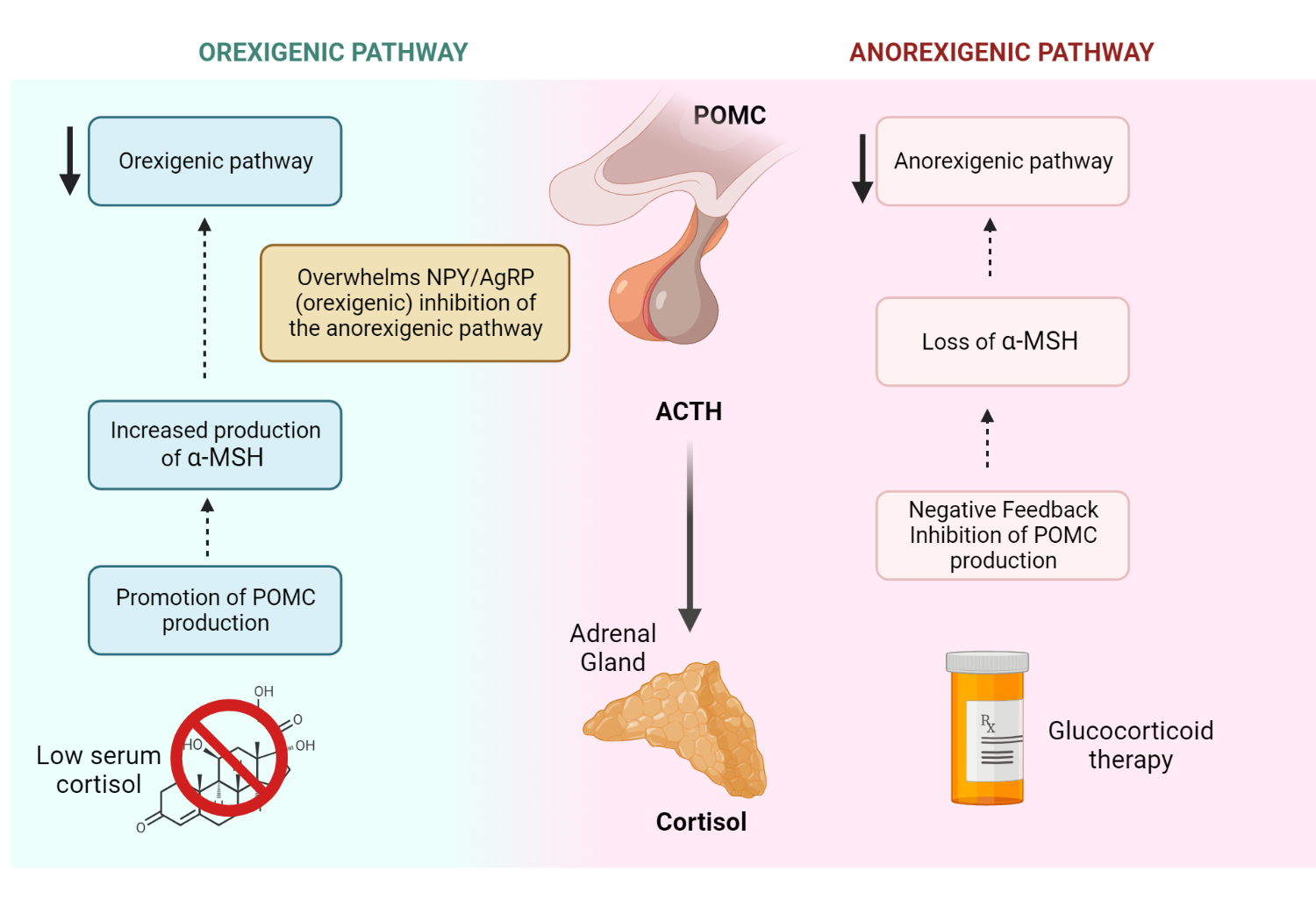
Why do patients with Addison’s disease have significant anorexia?
Hypocortisolemia in Addison’s disease lifts the normal negative feedback inhibition of POMC by cortisol. Significant overproduction of POMC, ACTH, and αMSH promotes the anorexigenic pathway, resulting in poor oral intake.
References
Song, NJ., Chang, SH., Li, D. et al. Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules. Exp Mol Med 49, e353 (2017).
Smitka K, Papezova H, Vondra K, Hill M, Hainer V, Nedvidkova J (2013) The Role of “Mixed” Orexigenic and Anorexigenic Signals and Autoantibodies Reacting with Appetite-Regulating Neuropeptides and Peptides of the Adipose Tissue-Gut-Brain Axis: Relevance to Food Intake and Nutritional Status in Patients with Anorexia Nervosa and Bulimia Nervosa. Int J Endocrinol 2013:483145
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM (1996) Leptin enters the brain by a saturable system independent of insulin. Peptides 17:305–311
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547
Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS (1997) Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138
Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578
Kristensen P, Judge ME, Thim L, et al (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76
Belgardt BF, Brüning JC (2010) CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 1212:97–113
Berthoud H-R (2002) Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26:393–428
Morton GJ, Meek TH, Schwartz MW (2014) Neurobiology of food intake in health and disease. Nat Rev Neurosci 15:367–378
Son JW, Kim S (2020) Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab J 44:802–818
Lonneman DJ, Rey JA, McKee BD (2013) Phentermine/Topiramate Extended-Release Capsules (Qsymia) for Weight Loss. P T 38:446–452
Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW (2011) Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377:1341–1352
Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW (2012) Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 20:330–342
Kindly Let Us Know If This Was helpful? Thank You!


