I. Overview of acromegaly
- Acromegaly is caused by tumors of somatotrophs that secrete excessive growth hormone (GH).
- In children, this results in gigantism, where they grow faster than normal, reaching heights of up to 2 m or more.
- In adults, linear growth is not possible due to the fusion of the epiphyses of the long bones. However, other tissues undergo hypertrophy, leading to a stereotypic appearance.
- In addition to physical changes, the metabolic effects of excess GH cause an increased risk of diabetes, hypertension, and vascular disease, leading to increased morbidity.
II. Characteristics of Acromegaly
- Acromegaly presents with coarse features, a prognathic jaw, and frontal bossing.
- Other features include increased jaw growth, leading to widely spaced teeth, and enlarged hands.
III. Diagnosis of Acromegaly
- Random blood GH concentration is usually not helpful in diagnosis due to its pulsatile secretion.
- For suspected cases, suppression of GH secretion should be performed using a glucose tolerance test. Unlike normal individuals, GH levels do not fall to zero in patients with acromegaly during this test.
- The final diagnostic test involves measuring the level of IGF1, which is a marker of long-term exposure to GH. This test is necessary because starvation can give a false positive response.
IV. Treatment of acromegaly
- Treatment options include surgery, radiation therapy, or drugs.
- Some patients respond to dopamine agonists, while more respond to somatostatin analogs such as lanreotide and octreotide.
- Surgery is often a more viable early option as somatotropinomas shrinkage does not typically occur with the medication.
- Following transsphenoidal surgery, the patient’s pituitary axes must be reassessed. If GH levels are low enough, no further therapy will be necessary.
- Some patients may progress to requiring radiotherapy and drugs.
- All patients with acromegaly require an annual pituitary review to ensure that they are on optimal pituitary hormone replacement therapy if needed.
Somatostatin analogs
Somatostatin analogs (SSA) activate somatostatin receptors on somatotroph tumor cells and thus inhibit their production of GH and, consequently, liver IGF-1. There is evidence that SSAs also lead to a reduction in tumor size secreting GH secreting tumors as well[1,2].
SSAs approved in the United States for the treatment of acromegaly include octreotide (short release), long-acting octreotide release (Sandostatin LAR), and lanreotide (somatuline). Cabergoline is more effective than bromocriptine and reduces tumor size in approximately 30% of patients[3].
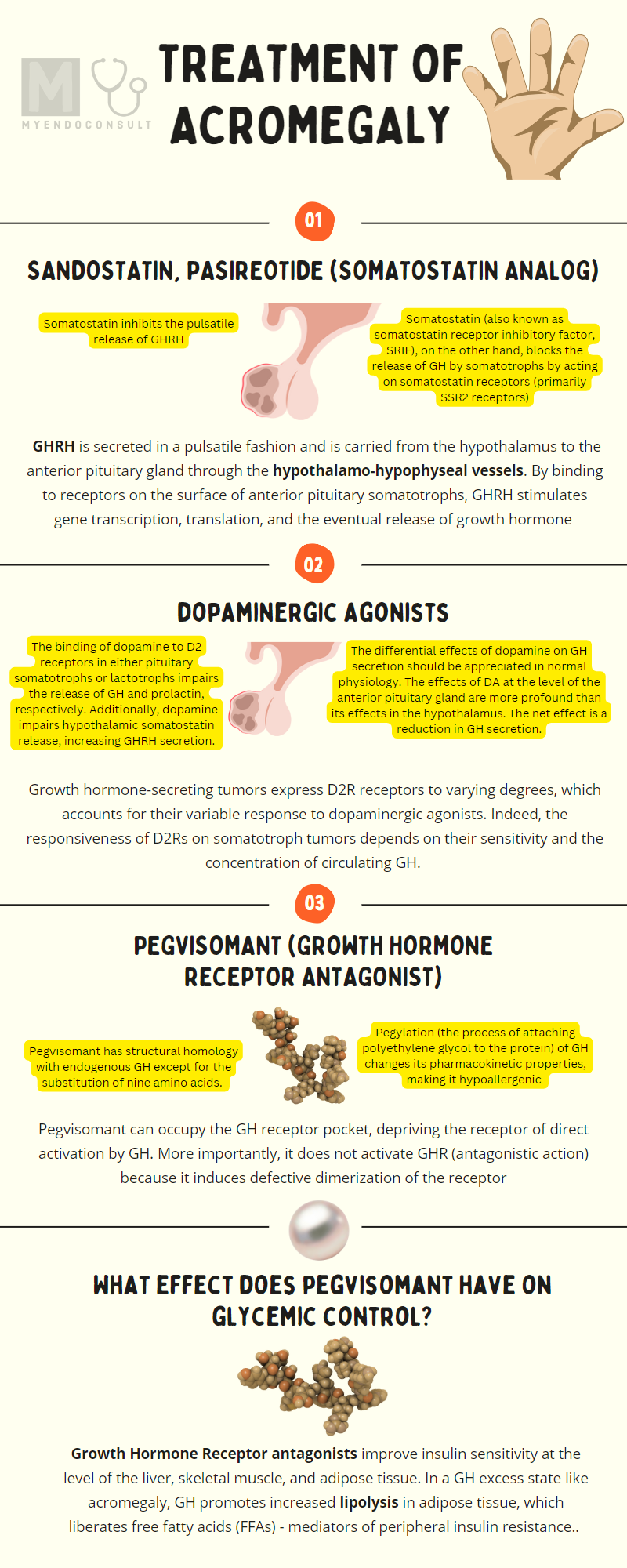
Growth hormone and insulin-like growth factor 1 axis
Hypothalamic-derived GHRH binds to GHRH-R in anterior pituitary somatotrophs and consequently mediates their secretion of GH. Binding of GH to GH-R in the liver promotes the synthesis of IGF-1, ALS, and IGFB3 – a complex that determines the circulating half-life and, therefore, peripheral effects of IGF-1. IGF-1 exerts negative feedback inhibition of GH and GHRH in the anterior pituitary and hypothalamus, respectively. GH exerts negative feedback inhibitory effects on its production at the level of the anterior pituitary gland. Somatostatin inhibits the release of growth hormones by somatotrophs. Important coinhibitory and co-stimulatory factors that influence net GH secretion are shown (negative and positive signs). Redrawn and modified from Ranke MB, Wit JM (2018) Growth hormone - past, present, and future. Nat Rev Endocrinol 14:285–300
- A hallmark side effect of pasireotide is hyperglycemia. The risk of hyperglycemia is disproportionately higher among patients with diabetes or prediabetes; it is reasonable to detect hyperglycemia before and during treatment [4].
- First-generation SSAs (for example, lanreotide and octreotide) have a higher affinity for SSR2 than the SSR5 isoform of the somatostatin receptor[5]. Pasireotide, on the other hand, has a higher affinity for the SSR5 receptor subtype. Tumor response is influenced by the density of specific histological subtypes of SSR (eg, sparse vs. densely granulated growth hormone-secreting tumors)[6]. This is clinically relevant as it can influence the effectiveness of selected therapies. For example, sparsely granulated somatotroph tumors tend to express SSR5 receptors (pasireotide). Densely granulated somatotrophs, on the other hand, express the SSR2 subtype (octreotide) predominantly [76–78].
- Somatostatin analogs are associated with an increased risk of cholelithiasis and diarrhea[7].
- Monitoring response to therapy – IGF-1 should normalize to the age- and sex-specific reference range for IGF-1. Furthermore, GH should be suppressed to a nadir of <1 ng / ml or <0.4ng/mL (for newer and more sensitive GH assays) after an oral glucose load with 75 grams of anhydrous glucose (oral glucose tolerance test).
Growth Hormone Receptor Antagonist (Pegvisomant)
Pegvisomant has structural homology to endogenous GH except for the substitution of nine amino acids. Pegylation (the process of attaching polyethylene glycol to the protein) of GH changes its pharmacokinetic properties, making it hypoallergenic[8].
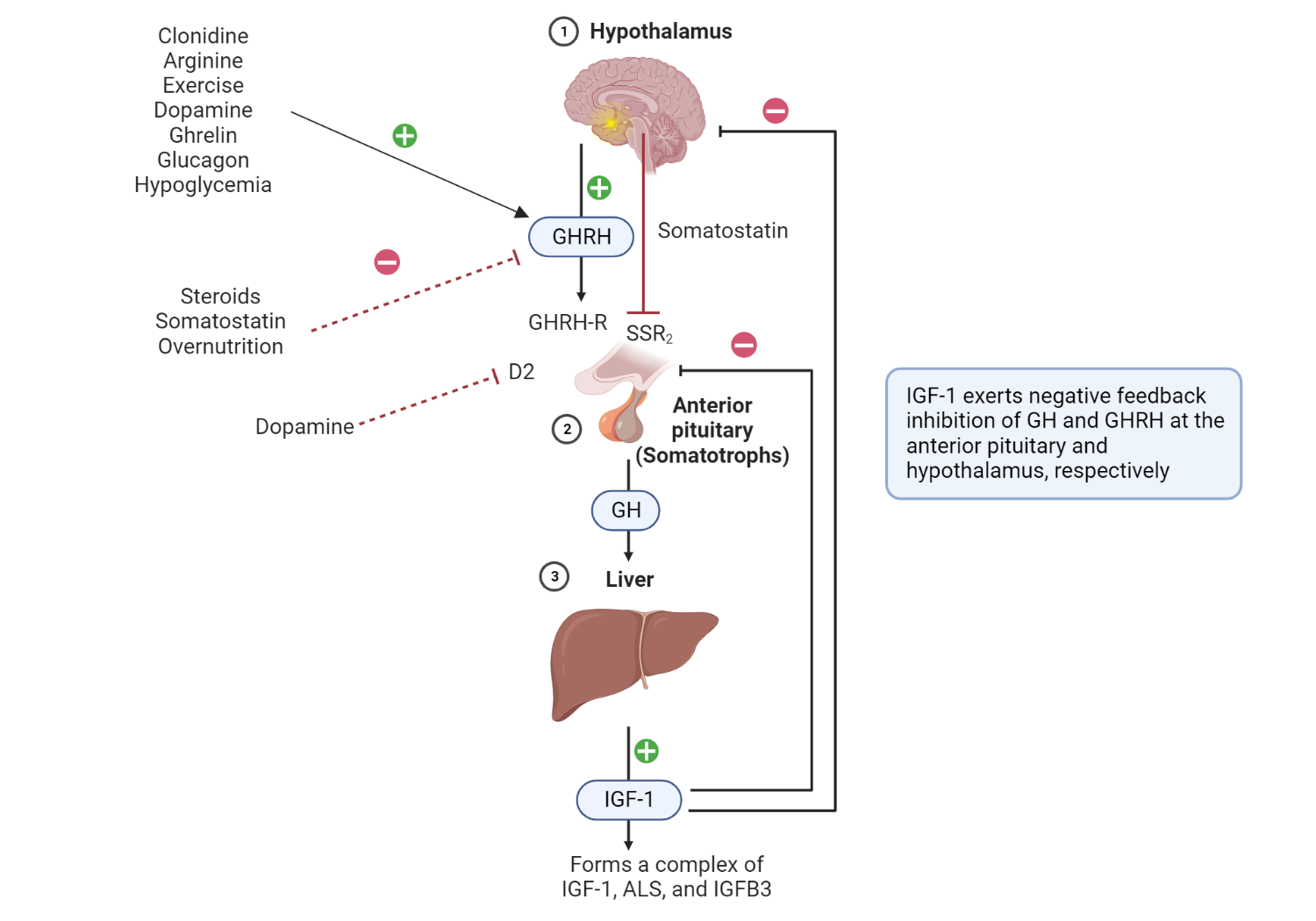
Due to its similarity to GH, Pegvisomant can occupy the GH receptor pocket, depriving the receptor of direct activation by GH. More importantly, it does not activate GHR (antagonistic action) because it induces defective receptor dimerization, thus preventing subsequent signal transduction pathways (JAK-STAT signaling) and eventual production of IGF-1 production[9].
Growth hormone receptor and intracellular effects of GH on GHR binding. GH binds to GHR, leading to dimerization and consequent structural change in the receptor. This critical step initiates the Janus-Kinase (JAK) signal transducer and transcription activator (STAT) intracellular signaling pathway. Phosphorylation of tyrosine residues in both the GHR transmembrane domain and STAT molecules is mediated by activated JAK2 tyrosine kinase[10]. There are various STAT molecules involved in specific intracellular processes. STAT5 (transcription factor), for example, is translocated from the cytoplasm to the nucleus and binds to DNA in specific DNA binding regions(DBRs)[11], which encode protein sequences[12]. Mitogen-activated protein kinase (MAPK) activation by JAK2 ultimately exerts genomic (gene expression) and nongenomic effects. GH mediates the production of IGF-1 in various tissues other than the liver, including growth plate chondrocytes, adipose tissue, and skeletal muscle[12]. Redrawn and adapted from Dehkhoda et al. (2018) The Growth Hormone Receptor: Mechanism of receptor activation, cell signaling, and Physiological Aspects. Front Endocrinol 9:35 [11]
Dopaminergic Agonists
The binding of dopamine to D2 receptors in pituitary somatotrophs or lactotrophs alters the release of GH and prolactin, respectively[13]. Furthermore, dopamine impairs hypothalamic somatostatin release, increasing GHRH secretion [48].
The differential effects of dopamine on GH secretion should be appreciated in normal physiology. The effects of DA at the level of the anterior pituitary gland are more profound than its effects in the hypothalamus. The net effect is a reduction in GH secretion.
Growth hormone-secreting tumors express D2R receptors to varying degrees, determining their response to dopaminergic agonists[15]. In fact, the responsiveness of D2Rs to somatotroph tumors depends on their sensitivity and the concentration of circulating GH[16].
References
Kindly Let Us Know If This Was helpful? Thank You!


