Recent advances in knowledge on androgen receptor activation has enhanced our understanding of the mechanism of action of androgen action. Knowing the importance of tissue conversion of circulating testosterone to estradiol and dihydrotestosterone has also enhanced our understanding of androgen’s actions in the body. Studies have shown that anabolic steroids administered in supraphysiological doses do have a positive effect on the size and strength of muscle. However, what seems to be unclear is the nature of anabolic action of androgens on the muscle. It is speculated that the anabolic action of androgens may involve mechanisms that are independent of the androgen receptor. The dose-response relationships of anabolic actions compared to the health risks of androgenic-anabolic steroids have generated much controversy. It is important to note that most of the adverse effects of androgenic anabolic steroids can be reversed, but some are permanent, especially in women and children. There is low incidence of acute fatal events associated with abuse of androgenic anabolic steroids. But then, the actual risk may either be under-reported or under-recognized. The disease risks and long-term consequences of androgenic-anabolic steroids to the endocrine system remain to be thoroughly evaluated.
Indications
Anabolic steroids are also known as androgenic steroids. They are basically synthetic derivatives of testosterone. Androgenic steroids are used both legally and illegally, but whatever the case, their usage is gaining popularity. There exist two distinct classes of anabolic steroids: 1) 17 alpha alkyl derivatives such as oxandrolone, fluoxymesterone, and oxymetholone; and 2) 17 beta ester derivatives, including testosterone cypionate, testosterone enanthate, testosterone heptylate, testosterone propionate, nandrolone decanoate, nandrolone phenpropionate, and dromostanolone. Nandrolone phenpropionate, classified as a C18 androgenic anabolic steroid, was among the earliest anabolic steroids to be used as a performance-enhancing drug by professional athletes during the 1960s. As a result, it was prohibited from the Olympics by the International Olympic Committee in 1974. All anabolic steroids are categorized as Schedule III drugs by the U.S. Drug Enforcement Administration [1, 2, 3].
The FDA has approved the use of anabolic steroids for several medical conditions, including primary hypogonadism, delayed puberty in boys (using testosterone enanthate), hypogonadotropic hypogonadism (using testosterone cypionate, enanthate, and undecanoate), deficiency of gonadotropin and luteinizing hormone-releasing hormone, dysfunction of the pituitary-hypothalamic axis caused by various tumors, injury, and radiation. Additionally, testosterone may be used to treat primary testicular failure in patients with cryptorchidism, orchitis, testicular torsion, vanishing testis syndrome, previous history of orchiectomy, Klinefelter syndrome, chemotherapeutic agents, toxic damage caused by alcohol use, and heavy metals.
Androgenic steroids have been used for several non-FDA-approved indications, including bone marrow stimulation in cases of leukemia, aplastic anemia, and kidney failure, as well as to promote growth and stimulate appetite. They have also been used to increase muscle mass in individuals with malignancy and acquired immunodeficiency syndrome. Additionally, anabolic steroid use is prevalent among athletes at all levels, including in sports such as bodybuilding, weightlifting, baseball, football, cycling, wrestling, and many others, as a means of enhancing their performance.
Mechanism of action of Testosterone and Anabolic Steroids
Endogenous androgen is responsible for the growth and development of male sex organs, as well as the maintenance of secondary sex characteristics. Both endogenous anabolic steroids, such as testosterone and dihydrotestosterone, and synthetic anabolic steroids, exert their effects by binding to androgen receptors and activating them. In skeletal muscle, anabolic steroids regulate the transcription of target genes that are involved in the accumulation of DNA in skeletal muscle, which is required for muscle growth [3, 4, 5].
In addition to their effects on skeletal muscle, anabolic steroids also upregulate and increase the number of androgen receptors, which enables increased training intensity and indirectly contributes to increased muscle size and strength. Furthermore, anabolic steroids have a stimulatory effect on the brain through their effects on various central nervous system neurotransmitters, as well as through antagonism of glucocorticoids and stimulation of the growth hormone-insulin-like growth factor-1 axis.
Nandrolone decanoate and nandrolone phenpropionate are both known for their increased ratio of anabolic activity compared to androgenic activity. Nandrolone decanoate is a slow-acting anabolic steroid that is specifically designed to increase muscle mass. It works by promoting nitrogen retention in muscles, which leads to an increase in muscle size. In addition, it can provide relief from joint pain by promoting collagen synthesis and enhancing bone mineralization. Nandrolone phenpropionate also promotes muscle growth and increases appetite, as well as stimulating the production of red blood cells.
Dromostanolone is a synthetic anabolic steroid that possesses anti-estrogenic properties and is approximately five times more potent than methyltestosterone. It is commonly used by bodybuilders to prepare for competition. Dromostanolone works by increasing the retention of nitrogen, phosphorus, and potassium, which results in increased protein anabolism and a decrease in the catabolism of amino acids. This leads to an increase in the density and hardness of muscles, which can be beneficial for bodybuilders looking to enhance their physical appearance.
Physiology of anabolic androgenic steroids
The anabolic androgenic effects of anabolic steroids are primarily mediated through the androgen receptor (AR)-signaling action. Androgen receptors are present in a wide variety of human tissues and organs, including the skeletal muscle, liver, prostate, central nervous system, and bone. The binding of anabolic steroids to androgen receptors in these tissues and organs leads to a range of effects, including an increase in protein synthesis and muscle mass, enhanced bone growth and density, and an increase in red blood cell production.
Anabolic steroids exert their effects through three main mechanisms:
- Direct binding to the androgen receptor (AR) – Anabolic steroids bind directly to the androgen receptor in various tissues, including muscle, bone, and the central nervous system, resulting in increased protein synthesis and muscle mass, enhanced bone growth and density, and other effects.
- Via dihydrotestosterone (DHT) – Some anabolic steroids, such as testosterone, can be converted into DHT by the enzyme 5-alpha-reductase. DHT is a more potent androgen than testosterone and can bind to the androgen receptor, leading to similar effects as direct binding to the receptor.
- Via estrogen receptors – Some anabolic steroids, such as testosterone, can be converted into estradiol by the enzyme aromatase. Estradiol can bind to estrogen receptors in various tissues and can contribute to some of the anabolic effects of anabolic steroids. However, excess estradiol can also lead to unwanted side effects, such as gynecomastia (male breast enlargement) and water retention.
The transportation of free testosterone to the cytoplasm of target tissue cells leads to its binding with the AR. This binding can occur either directly or through the conversion of testosterone to 5αdihydrotestosterone (DHT) by the cytoplasmic enzyme 5-alpha reductase. Once inside the cell nucleus, both free and bound testosterone binds to specific nucleotide sequences of the chromosomal DNA. This binding activates the transcription of particular responsive genes that have a significant impact on protein synthesis [6, 7, 8].
Following dimerization, the complex binds to androgen response elements (AREs), which are specific promoter regions of target genes, and this interaction affects the transcription process [9]. In addition, rapid steroid hormone activation can occur through non-genomic pathways, which involve the interference of the steroid hormone with the G-protein coupled receptor. This receptor is a transmembrane receptor located inside the cell [10, 11]. Androgen receptors expressed in thyroid tissue may lead to thyrocyte proliferation in culture, independent of TSH, as a result of the influence of sex steroids on thyroid function [12]. Similar mechanisms have been described in other tissues as well [13].
Pathophysiology of anabolic androgenic steroids
The primary mechanisms that contribute to an increase in the circulation of anabolic-androgenic steroids (AASs) include the administration of synthetic derivatives of testosterone, the administration of testosterone itself, and the use of drugs that promote the production of endogenous testosterone [6]. When used in supraphysiological doses, the mechanism of action of anabolic-androgenic steroids (AASs) is marked by a disruption in testosterone biosynthesis within tissues.
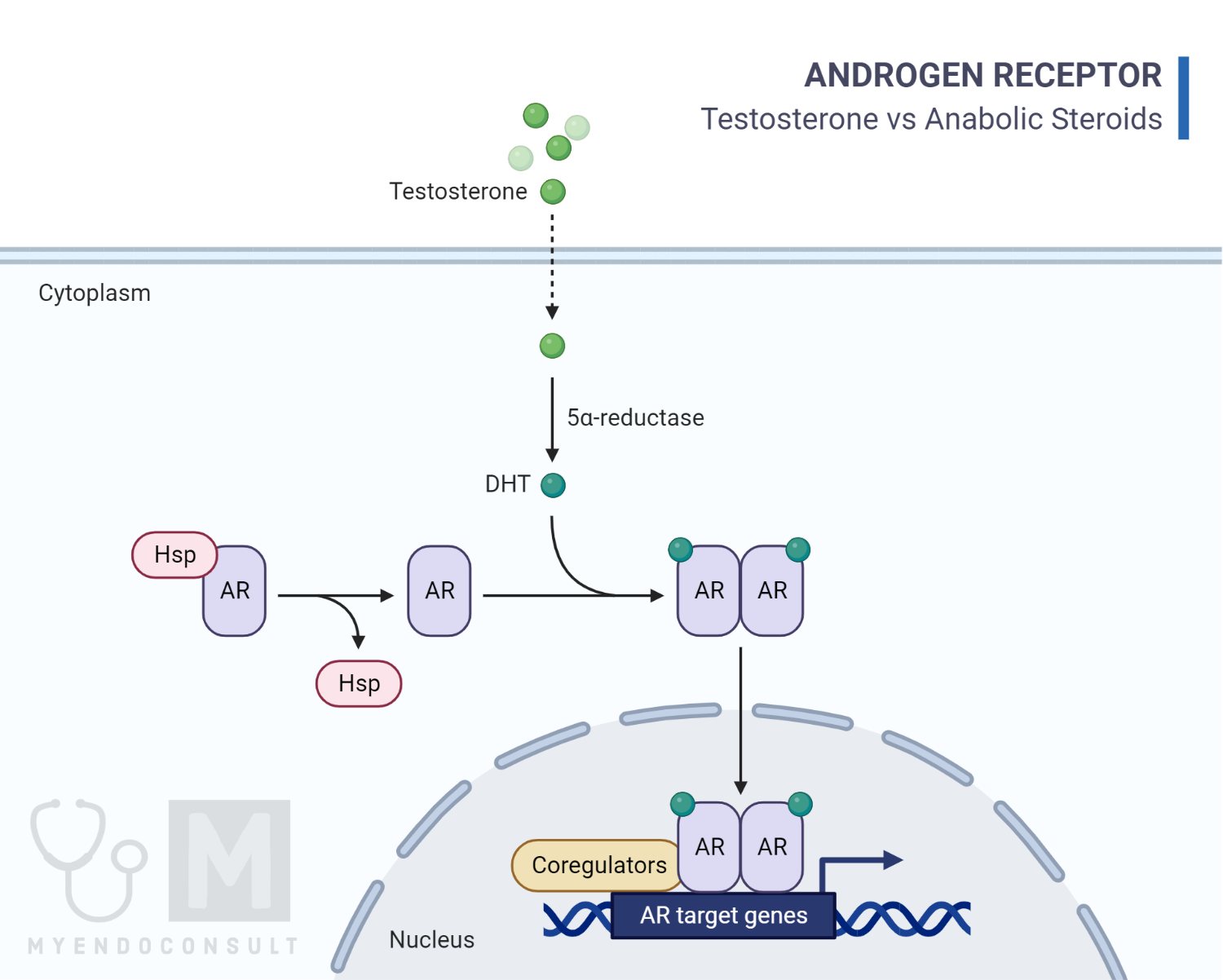
Fig 1: A diagram illustrating how anabolic steroids act within the cell.
Image credit: Image is based on Albano et al [14].
The effects of anabolic-androgenic steroids (AASs) are mediated through the activation of androgen receptor (AR) signaling. Due to the widespread expression of ARs in many different tissues, various parts of the body are involved in the response to AASs [7]. Under normal physiological conditions, androgen receptors (ARs) are typically saturated with testosterone, and any effects of anabolic-androgenic steroids (AASs) may not necessarily be solely due to AR activation. At high levels of testosterone, an antagonistic effect on glucocorticoid receptors may occur, resulting in the inhibition of glucose synthesis and protein catabolism. In fact, high doses of AASs may displace glucocorticoids from their receptors, reduce protein breakdown in muscles, and ultimately result in an increase in muscle mass and strength [15]. In addition to displacing glucocorticoids from their receptors, the inhibition of glucocorticoid action by AASs is also due to the stimulation of the growth hormone (GH) and insulin-like growth factor (IGF)-1 axis. AASs induce an androgen-mediated stimulation of GH and the hepatic synthesis of IGF-1, ultimately leading to the formation of muscle proteins and resulting in anabolic effects [16]. Testosterone is also converted to estradiol and estrone via aromatase action, which can impact brain and sexual differentiation, increase bone and muscle mass, and influence puberty and sexual functions. At high doses, AASs can have an antiestrogenic effect due to down-regulation of androgen receptors and competition with estrogens for their receptors [15].
Overall, the effects of anabolic-androgenic steroids (AASs) are the result of the amplification of the physiological consequences of both testosterone and estrogens. This is achieved through a variety of mechanisms, including the activation of androgen receptors, inhibition of glucocorticoid action, and stimulation of the GH/IGF-1 axis. The net result is an increase in muscle mass and strength, bone density, and alterations in sexual differentiation and function.
Numerous studies have suggested that anabolic-androgenic steroids (AASs) can have an impact on oxidative stress within the body. Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the ability of the body’s antioxidant systems to neutralize them. AAS use has been linked to an increase in ROS production, which can lead to oxidative damage to tissues and organs. This, in turn, may contribute to the development of various health problems associated with AAS use, including cardiovascular disease and liver damage [15, 17, 18].
Apoptosis, which is a form of programmed cell death, has also been linked to the damage caused by anabolic-androgenic steroids (AASs) [19, 20, 21]. Studies have shown that supraphysiological concentrations of anabolic-androgenic steroids (AASs) can induce neurotoxicity by involving the apoptotic process and neurodegeneration [20].
Administration of anabolic steroids
Anabolic steroids can be administered in various ways, including oral pills, injections, creams or topical gels, and skin patches. Each method has its own advantages and disadvantages, and the choice of administration route will depend on several factors, including the desired effect, the specific steroid being used, and the individual’s preferences and needs. For example, oral pills are easy to use and convenient but may have a higher risk of liver toxicity, while injections can provide more stable blood levels but may carry a risk of infections or injection site reactions.
- Testosterone cypionate is an injectable form of testosterone that is commonly used for the treatment of primary hypogonadism and hypogonadotropic hypogonadism in males. The recommended dosage of testosterone cypionate for this purpose is typically in the range of 50 to 400 mg, given intramuscularly once every two to four weeks.
- Transdermal testosterone is applied topically to the skin. The recommended dosage of transdermal testosterone may vary depending on the specific product being used, but typically ranges from 50 to 100 mg applied once daily to the upper limb, shoulder, or abdomen.
- Testosterone gel is administered as 11mg thrice daily, with a daily total dose of 33mg.
- The dosing regimen for testosterone undecanoate involves an initial dose of 750 mg, followed by another 750 mg dose given four weeks after the first dose. Subsequently, 750 mg doses are administered at ten-week intervals between each dose.
Non-FDA approved medications
- For the purpose of providing comfort and relief of joint pain, the recommended dosing regimen for nandrolone decanoate is 100 mg per week. However, for individuals seeking to increase growth and performance, the dosage can be increased to the range of 200-400 mg per week. It is typically recommended to use nandrolone decanoate for a period of ten to twelve weeks to achieve desired results, especially in the case of athletes, powerlifters, and bodybuilders.
- Dromostanolone is typically available in doses of 200 to 400 mg per week, which bodybuilders use to enhance their athletic performance. Due to its relatively short half-life, dromostanolone injections are typically administered every three to four days.
References
- Lusetti M, Licata M, Silingardi E, Bonsignore A, Palmiere C. Appearance/Image- and Performance-Enhancing Drug Users: A Forensic Approach. Am J Forensic Med Pathol. 2018;39(4):325-329. doi:10.1097/PAF.0000000000000424
- Jones IA, Togashi R, Hatch GFR 3rd, Weber AE, Vangsness CT Jr. Anabolic steroids and tendons: A review of their mechanical, structural, and biologic effects. J Orthop Res. 2018;36(11):2830-2841. doi:10.1002/jor.24116
- Armstrong JM, Avant RA, Charchenko CM, et al. Impact of anabolic androgenic steroids on sexual function. Transl Androl Urol. 2018;7(3):483-489. doi:10.21037/tau.2018.04.23
- Melo Junior AF, Dalpiaz PLM, Sousa GJ, et al. Nandrolone alter left ventricular contractility and promotes remodelling involving calcium-handling proteins and renin-angiotensin system in male SHR. Life Sci. 2018;208:239-245. doi:10.1016/j.lfs.2018.07.041
- Zhou S, Glowacki J. Dehydroepiandrosterone and Bone. Vitam Horm. 2018;108:251-271. doi:10.1016/bs.vh.2018.01.005
- Anawalt BD. Diagnosis and Management of Anabolic Androgenic Steroid Use. J Clin Endocrinol Metab. 2019;104(7):2490-2500. doi:10.1210/jc.2018-01882
- Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154(3):502-521. doi:10.1038/bjp.2008.165
- Sessa F, Salerno M, Di Mizio G, et al. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front Pharmacol. 2018;9:1321. Published 2018 Nov 20. doi:10.3389/fphar.2018.01321
- Alsiö J, Birgner C, Björkblom L, et al. Impact of nandrolone decanoate on gene expression in endocrine systems related to the adverse effects of anabolic androgenic steroids. Basic Clin Pharmacol Toxicol. 2009;105(5):307-314. doi:10.1111/j.1742-7843.2009.00439.x
- Joseph JF, Parr MK. Synthetic androgens as designer supplements. Curr Neuropharmacol. 2015;13(1):89-100. doi:10.2174/1570159X13666141210224756
- Cheung AS, Grossmann M. Physiological basis behind ergogenic effects of anabolic androgens. Mol Cell Endocrinol. 2018;464:14-20. doi:10.1016/j.mce.2017.01.047
- Fortunato RS, Marassi MP, Chaves EA, Nascimento JH, Rosenthal D, Carvalho DP. Chronic administration of anabolic androgenic steroid alters murine thyroid function. Med Sci Sports Exerc. 2006;38(2):256-261. doi:10.1249/01.mss.0000183357.19743.51
- Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord. 2015;16(3):199-211. doi:10.1007/s11154-015-9320-5
- Albano GD, Amico F, Cocimano G, et al. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare (Basel). 2021;9(1):97. Published 2021 Jan 19. doi:10.3390/healthcare9010097
- Arazi H, Mohammadjafari H, Asadi A. Use of anabolic androgenic steroids produces greater oxidative stress responses to resistance exercise in strength-trained men. Toxicol Rep. 2017;4:282-286. Published 2017 Jun 8. doi:10.1016/j.toxrep.2017.05.005
- El Osta R, Almont T, Diligent C, Hubert N, Eschwège P, Hubert J. Anabolic steroids abuse and male infertility. Basic Clin Androl. 2016;26:2. Published 2016 Feb 6. doi:10.1186/s12610-016-0029-4
- Frankenfeld SP, Oliveira LP, Ortenzi VH, et al. The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male Wistar rats. PLoS One. 2014;9(9):e102699. Published 2014 Sep 16. doi:10.1371/journal.pone.0102699
- Frankenfeld SP, de Oliveira LP, Ignacio DL, et al. Nandrolone decanoate inhibits gluconeogenesis and decreases fasting glucose in Wistar male rats. J Endocrinol. 2014;220(2):143-153. Published 2014 Jan 8. doi:10.1530/JOE-13-0259
- Turillazzi E, Neri M, Cerretani D, et al. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J Cell Mol Med. 2016;20(4):601-612. doi:10.1111/jcmm.12748
- Basile JR, Binmadi NO, Zhou H, Yang YH, Paoli A, Proia P. Supraphysiological doses of performance enhancing anabolic-androgenic steroids exert direct toxic effects on neuron-like cells. Front Cell Neurosci. 2013;7:69. Published 2013 May 9. doi:10.3389/fncel.2013.00069
- D’Errico S, Di Battista B, Di Paolo M, Fiore C, Pomara C. Renal heat shock proteins over-expression due to anabolic androgenic steroids abuse. Mini Rev Med Chem. 2011;11(5):446-450. doi:10.2174/138955711795445934
Kindly Let Us Know If This Was helpful? Thank You!


