What is hypogonadism ?
Hypogonadism in a male is defined as a decrease in one or both of the two major functions of the testes, that is spermiogensis (sperm production) or androgen (testosterone) production.
Hypogonadism can result from either an insult to the testes (primary hypogonadism) or the hypothalamus or pituitary (secondary hypogonadism).
Comparison of primary vs secondary hypogonadism
| Primary hypogonadism | Secondary hypogonadism |
| Serum testosterone concentration and sperm count are below normal | Serum testosterone concentration and the sperm count are also below normal |
| Serum LH and FSH concentrations are above normal. | Serum LH and FSH concentrations are normal or reduced. |
| More likely to be associated with a decrease in sperm production than in testosterone production. | Secondary hypogonadism is associated with a proportionate reduction in testosterone and sperm production. |
| Testes is usually small (measured with an orchidometer)** | Testes may be normal in size |
**Although many testicular diseases damage both the seminiferous tubules and the Leydig cells, they usually damage the seminiferous tubules to a greater degree.
Causes of primary hypogonadism
Causes of primary hypogonadism in males can include congenital abnormalities and acquired diseases.
Congenital abnormalities:
- Klinefelter syndrome (and other chromosomal abnormalities)
- Mutation in the FSH and LH receptor genes
- Cryptorchidism
- Varicocele
- Disorders of androgen synthesis, and
- Myotonic dystrophy.
Acquired diseases:
- Infections (especially mumps)
- Radiation
- Alkylating agents
- Ketoconazole
- Glucocorticoids
- Environmental toxins
- Trauma
- Testicular torsion
- Autoimmune damage
- Chronic systemic illnesses
- Hepatic cirrhosis
- Chronic renal failure
- AIDS
Causes of secondary hypogonadism
Causes of secondary hypogonadism in males can include congenital abnormalities and acquired diseases.
Congenital :
- Isolated GnRH deficiency (without anosmia, Kallman syndrome or associated with adrenal hypoplasia congenita)
- GnRH deficiency with mental challenges (e.g., Prader-Willi syndrome)
- Idiopathic anterior pituitary insufficiency
- Congenital malformations with craniofacial anomalies
Acquired:
- Brain Tumors (craniopharyngiomas, meningiomas, gliomas, germinomas, astrocytomas)
- Functional gonadotropin deficiency (malnutrition, acute illness, chronic systemic disease, eating disorders, post-anabolic steroid abuse, hypothyroidism, prolactinoma, Cushing’s disease)
- Infiltrative conditions (histiocytosis X, granulomatous diseases, hemochromatosis)
- Pituitary apoplexy
- Illicit drugs (opioids, cannabinoids, anabolic steroids)
Klinefelter’s syndrome vs Kallmann’s syndrome
| Klinefelter’s syndrome | Kallmann’s syndrome |
| hypergonadotropic hypogonadism | hypogonadotrophic hypogonadism |
| No anosmia | Often associated with anosmia. |
| At risk for autoimmune diseases | No risk for autoimmune disease |
| Poor academic record | Normal intelligence |
What are the clinical features of Klinefelter’s syndrome
Kleinefelter’s syndrome (KS) is a congenital abnormality that causes primary hypogonadism. KS occurs during meiotic division when there is non-disjunction of either parent’s sex hormones resulting in a 47, XXY genotype.
Clinical features include:
- Small and firm testes
- Low sperm count and infertility
- Eunuchoid Stature
- Gynaecomastia
Patients are also more susceptible to various autoimmune diseases such as systemic lupus erythematosus, diabetes mellitus, rheumatoid arthritis, and Sjogren’s syndrome.
Also, atrophy and damage to the seminiferous tubules and Leydig cells lead to low testosterone levels and high gonadotropin (FSH and LH) levels.
KS is diagnosed based on karyotyping. Androgen replacement is required to maintain quality of life, muscle development, secondary sexual characteristics, and bone strength. Furthermore, some patients can have their own children using a combination of testicular sperm extraction and IVF techniques, such as intracytoplasmic sperm injection.
The hypothalamic Pituitary Testicular Axis
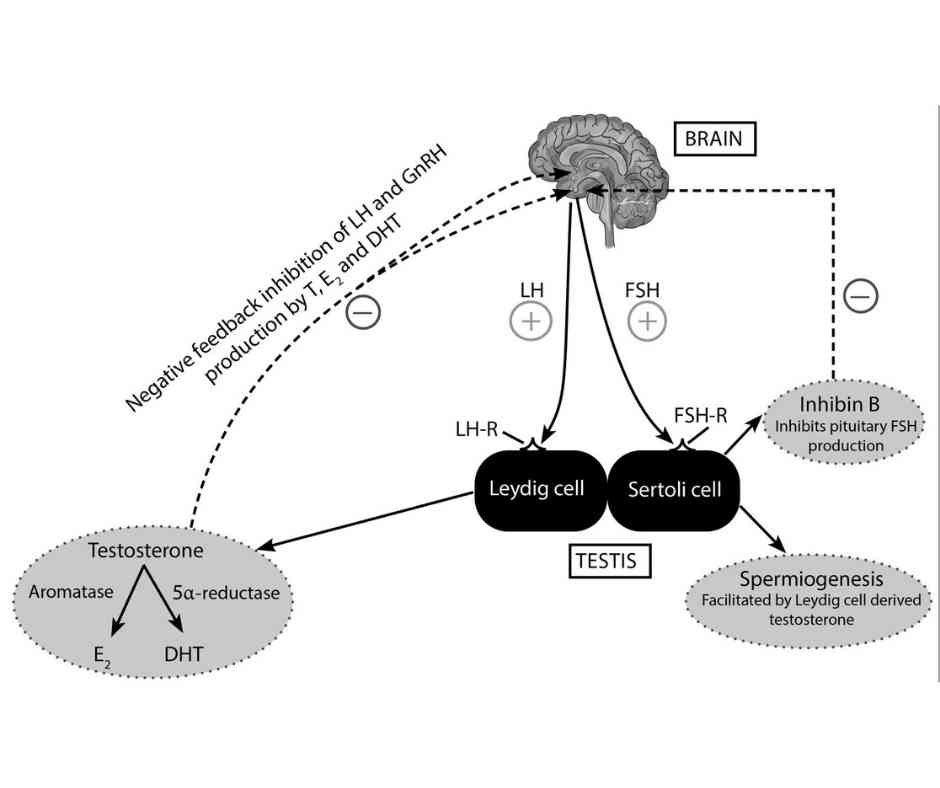
Testosterone is produced by Leydig cells of the testes under trophic stimulation by LH (produced by the anterior pituitary gland).
Sperms are produced by seminiferous tubules under stimulation by a high intra-testicular concentration of testosterone. Also, FSH acts on Sertoli cells of the seminiferous which promotes the production of both sperm and inhibin B.
Extratesticular testosterone for patients on exogenous testosterone therapy does not increase sperm production. Indeed, testosterone monotherapy reduces sperm count and renders patients temporarily infertile.
Circulating testosterone inhibits both LH and FSH secretion. FSH production is also inhibited by inhibin B, a product of the Sertoli cells of the seminiferous tubules.
Kindly Let Us Know If This Was helpful? Thank You!


