They are a complex composition of cholesterol esters and triacylglycerols in a dense lipid core, with a circumferential rim of free cholesterol, apolipoproteins, and phospholipids. This intricate configuration allows water-insoluble cholesterol and triacylglycerols to be ferried through the circulatory system to their target tissues[1, 2].
The classification of lipoproteins depends on their size, lipid composition, and specific apolipoprotein subtype. There are various lipoproteins, including low-density lipoprotein (LDL), high-density lipoproteins (HDL), very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL) and chylomicrons[3].
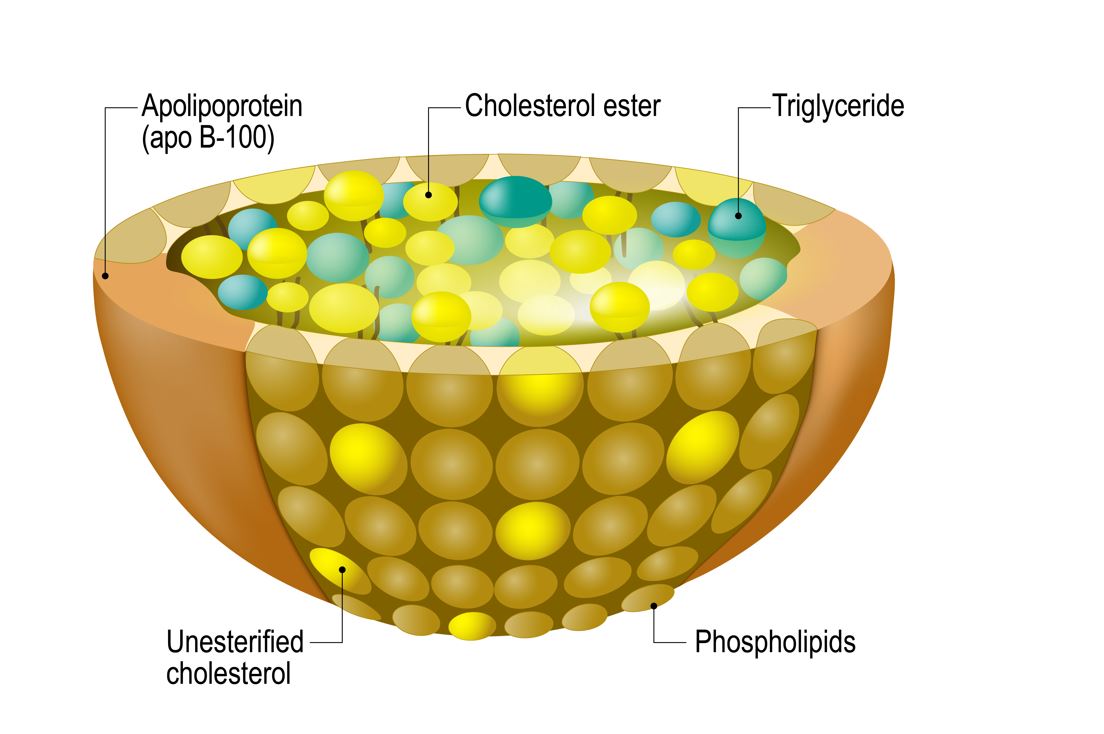
Figure 1 Structure of lipoproteins.
What are apoproteins/apolipoproteins
These complex proteins present on the surface of lipoproteins play critical roles in lipoprotein physiology by providing structural integrity to lipoproteins, modulating lipoprotein-related enzymatic action, and serving as ligands for various lipoproteins at specific target tissues[4].
Lipoprotein metabolism
Lipoprotein metabolism aims to transfer dietary sources of triglycerides to both muscle and adipose tissue for energy metabolism and storage, respectively. It also facilitates the cycling of cholesterol between the liver and peripheral tissues, a process critical for the formation of steroid hormones, cell membranes, and bile acids[5].
Exogenous and endogenous lipoprotein pathway
Step 1: Bile salts emulsify fat globules into smaller fatty droplets that contain triglycerides (TAGs) and cholesterol esters (CEs). Dietary triglycerides are then hydrolyzed into free fatty acids (FFAs) and monoacylglycerol (MAG) in the intestinal lumen by pancreatic lipases. This initial step enables the eventual transfer of TAGs from the intestinal lumen into the circulatory system.
The products of hydrolysis of TAGs, i.e., FFAs and MAG, are then repackaged into micelles, which then diffuse into the cytosol of the enterocyte[5, 6]. Once in the enterocyte, FFAs and MAG are then resynthesized into TAGs in preparation for delivery to the circulatory system. TAGs, cholesterol esters, cholesterol, and apolipoprotein B-48 (Apo B-48) are then packaged into chylomicrons in the enterocyte[7]. This concludes the formation of the first lipoprotein in the exogenous lipoprotein pathway[8].
Step 2: Chylomicrons enter systemic circulation via the thoracic duct, thus bypassing the portal circulation [6, 9]. Once in the systemic circulation, HDL transfers apolipoprotein CII (Apo-CII) and apolipoprotein E (Apo-E) to chylomicrons. Apo-E allows the binding of lipoproteins to specific LDL receptors or LDL-like receptors present in various tissues (liver and adrenal cortex). Apo-CII, on the other hand, is critical in activating the lipoprotein lipase, present on the capillary endothelium[10].
Step 3: Chylomicrons reach adipose tissue and muscle, where lipoprotein lipase (produced by adipocytes and muscle cells) present on the endothelial lining of capillaries hydrolyzes TAGs into FFAs and monoacylglycerol. FFAs are then taken up by adipocytes and muscle cells for energy metabolism[5]. Chylomicron remnants, which are formed after this step, then release the previously acquired apo-CII, which was needed for lipoprotein lipase activity, back to HDL[11]. This concludes the exogenous lipoprotein pathway, which facilitates the transfer of dietary sources of fatty acids from the intestine to muscle and adipose tissue, for both storage and metabolism[12].
Step 4: Chylomicron remnants, which contain mostly cholesterol and cholesterol esters, bind to the hepatic LDL receptor and are transported into the liver[13].
Step 5: Once in the liver, TAGs, cholesterol esters, and Apo-B100 are repackaged into VLDL. VLDL in circulation, just like chylomicrons, receives Apo-E and Apo-CII from HDL. VLDL is transported to adipose tissue and the muscle, where again, lipoprotein lipase facilitates the hydrolysis of TAGs into FFAs and monoacylglycerol. FFAs can then be stored in fatty tissue or used for energy metabolism by muscle. The loss of TAGs from VLDL results in the formation of intermediate-density lipoproteins (IDL) or VLDL remnants. Again, Apo-CII will be released back to HDL after the formation of IDL[10, 14].
Step 6: IDL in circulation, with its surface bound Apo-E, has an affinity for tissues with LDL or LDL-like receptors. IDL binds to the hepatic LDL receptor, where it is taken up by the liver for further processing. Apo-B100 and cholesterol esters are then repackaged into a new lipoprotein called LDL[10].
Step 7: LDL binds LDL-like receptors in the gonads for gonadal steroidogenesis[15] and the adrenal cortex for adrenal steroidogenesis[16, 17]. LDL can also bind LDL receptors present in muscle and adipose tissue, where it can be taken up for further processing. Ultimately, LDL tracks back to the liver to conclude the endogenous lipoprotein pathway, whose primary purpose is to transfer cholesterol and TAGs from the liver to peripheral tissues[18]. Figure 2 depicts critical steps in the lipoprotein synthesis pathway.
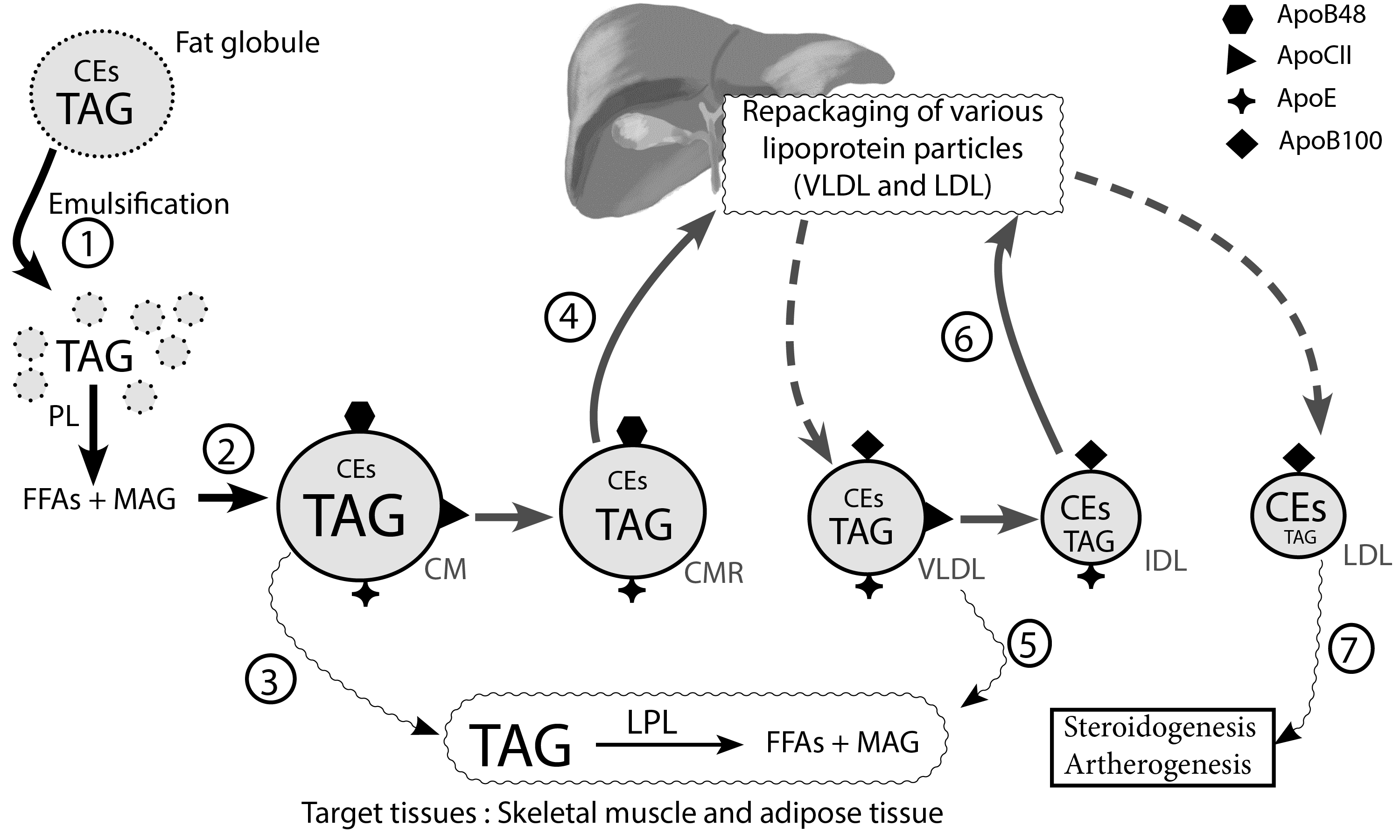
Figure 2 Exogenous and endogenous lipoprotein pathway. Emulsification of fat globules into tiny fat droplets by bile (step 1)[5–7], facilitates the hydrolysis of triacylglycerides (TAG) into free fatty acids (FFAs) and monoacylglycerol (MAG) by pancreatic lipase (PL) in the intestinal lumen. Chylomicron (CM) formed from repackaged TAG, cholesterol esters (CEs), and various apoproteins (step 2)[6, 9, 10] are delivered to target tissues where TAG is hydrolyzed by lipoprotein lipase (LPL) (step 3)[5, 11, 12]. Chylomicron remnants (CMR) are transported to the liver for further processing (step 4)[13]. VLDL released from the liver is destined for adipose tissue and skeletal muscle, where stores of TAG are released for hydrolysis by LPL(step 5)[10, 14]. IDL formed after VLDL processing in target tissues is destined for the liver (step 6)[10]. LDL, a cholesterol ester (CE) rich lipoprotein, is critical in steroidogenesis in various tissues and plays a central role in atherogenesis(step 7)[16–18] (Redrawn and modified from Ramasamy I (2014) Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med 52:1695–1727)
Reverse cholesterol transport pathway
HDL is responsible for transferring cholesterol and triglycerides from peripheral tissues back to the liver[19] and finally into the intestine for excretion[20].
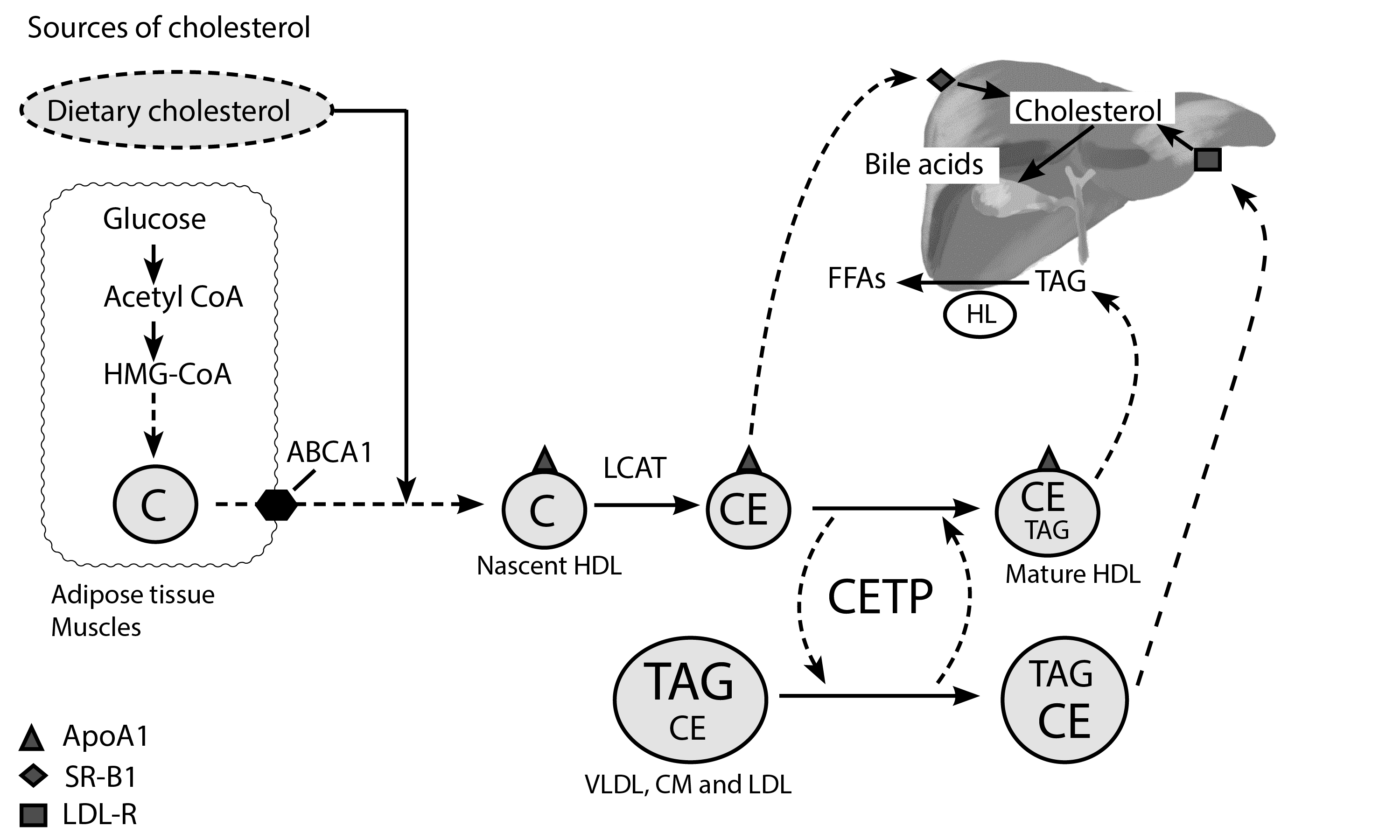
Figure 3. Schematic representation of reverse cholesterol transport pathway. Free cholesterol from extrahepatic tissues (muscles and adipose tissue) is transferred to ApoA-1 to form the nascent HDL particle; this process is facilitated by ATP-binding cassette transporter A1 (ABCA1) present on the cell membrane of extrahepatic tissues (muscles, adipose tissue, and intestine). Cholesterol (C) is then converted to cholesterol esters by Lecithin cholesterol acyltransferase (LCAT), which is critical in the formation of mature HDL[10]. Cholesterol ester transfer protein (CETP) is essential in the transfer of cholesterol esters and triglycerides between other circulating lipoproteins (chylomicrons, VLDL, LDL) and HDL. CETP transfers CE’s from HDL to other lipoproteins and carries TAGs from these lipoproteins back to the mature HDL particle. HDL, therefore, becomes rich in TAGs at the expense of CEs [21]. HDL binds to the hepatic scavenger SR-B1 receptor, where it releases its cholesterol esters without being internalized by the liver. It then returns to circulation as a smaller HDL particle, to repeat the process of cholesterol and triglyceride acquisition [22, 23]. HDL may also be hydrolyzed by hepatic lipase (HL) into FFAs, converting it back to a smaller HDL particle, which can then be recycled in the process of cholesterol acquisition. HDL can also bind the hepatic LDL-R receptor[24], a process that facilitates the release of cholesterol to the liver for bile acid synthesis[25]. (Redrawn and modified from Ramasamy I (2014) Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med 52:1695–1727)
https://youtu.be/JckJg4hytyE
References
- Ginsberg HN (1990) Lipoprotein physiology and its relationship to atherogenesis. Endocrinol Metab Clin North Am 19:211–228
- Pullinger CR, Kane JP (2006) Lipid and Lipoprotein Metabolism. Reviews in Cell Biology and Molecular Medicine. https://doi.org/10.1002/3527600906.mcb.200400101
- Ginsberg HN (1998) Lipoprotein physiology. Endocrinol Metab Clin North Am 27:503–519
- Alaupovic P (2003) The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr Atheroscler Rep 5:459–467
- Ramasamy I (2014) Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med 52:1695–1727
- Hussain MM (2014) Intestinal Lipid Absorption and Lipoprotein Formation. Curr Opin Lipidol 25:200–206
- Iqbal J, Hussain MM (2009) Intestinal lipid absorption. Am J Physiol Endocrinol Metab 296:E1183–E1194
- Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT (2017) Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 267:49–60
- Kindel T, Lee DM, Tso P (2010) The mechanism of the formation and secretion of chylomicrons. Atheroscler Suppl 11:11–16
- Cohen DE, Fisher EA (2013) Lipoprotein Metabolism, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Semin Liver Dis 33:380–388
- Cooper AD (1997) Hepatic uptake of chylomicron remnants. J Lipid Res 38:2173–2192
- Daniels TF, Killinger KM, Michal JJ, Wright Jr. RW, Jiang Z (2009) Lipoproteins, cholesterol homeostasis and cardiac health. Int J Biol Sci 5:474–488
- Willnow TE (1997) Mechanisms of hepatic chylomicron remnant clearance. Diabet Med 14 Suppl 3:S75-80
- Beisiegel U (1998) Lipoprotein metabolism. Eur Heart J 19 Suppl A:A20-23
- Hu J, Zhang Z, Shen W-J, Azhar S (2010) Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond) 7:47
- Miller WL, Bose HS (2011) Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135
- Bochem AE, Holleboom AG, Romijn JA, Hoekstra M, Dallinga-Thie GM, Motazacker MM, Hovingh GK, Kuivenhoven JA, Stroes ESG (2013) High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: a study in individuals with low plasma HDL-C. J Lipid Res 54:1698–1704
- Geldenhuys WJ, Lin L, Darvesh AS, Sadana P (2017) Emerging strategies of targeting lipoprotein lipase for metabolic and cardiovascular diseases. Drug Discov Today 22:352–365
- Tall AR (1998) An overview of reverse cholesterol transport. Eur Heart J 19 Suppl A:A31-35
- Lin X, Racette SB, Ma L, Wallendorf M, Ostlund RE (2017) Ezetimibe Increases Endogenous Cholesterol Excretion in Humans. Arterioscler Thromb Vasc Biol 37:990–996
- Tall AR (2010) Functions of cholesterol ester transfer protein and relationship to coronary artery disease risk. J Clin Lipidol 4:389–393
- Zhou L, Li C, Gao L, Wang A (2015) High-density lipoprotein synthesis and metabolism (Review). Molecular Medicine Reports 12:4015–4021
- Afonso MS, Machado RM, Lavrador MS, Quintao ECR, Moore KJ, Lottenberg AM (2018) Molecular Pathways Underlying Cholesterol Homeostasis. Nutrients 10:760
- Trajkovska KT, Topuzovska S (2017) High-density lipoprotein metabolism and reverse cholesterol transport: strategies for raising HDL cholesterol. Anatol J Cardiol 18:149–154
- Staels B, Fonseca VA (2009) Bile Acids and Metabolic Regulation. Diabetes Care 32:S237–S245
Kindly Let Us Know If This Was helpful? Thank You!



Glad this was helpful.
Thanks so much!!