Clonidine Suppression Test (Infographic)
Physiology and Rationale of the Clonidine Suppression Test
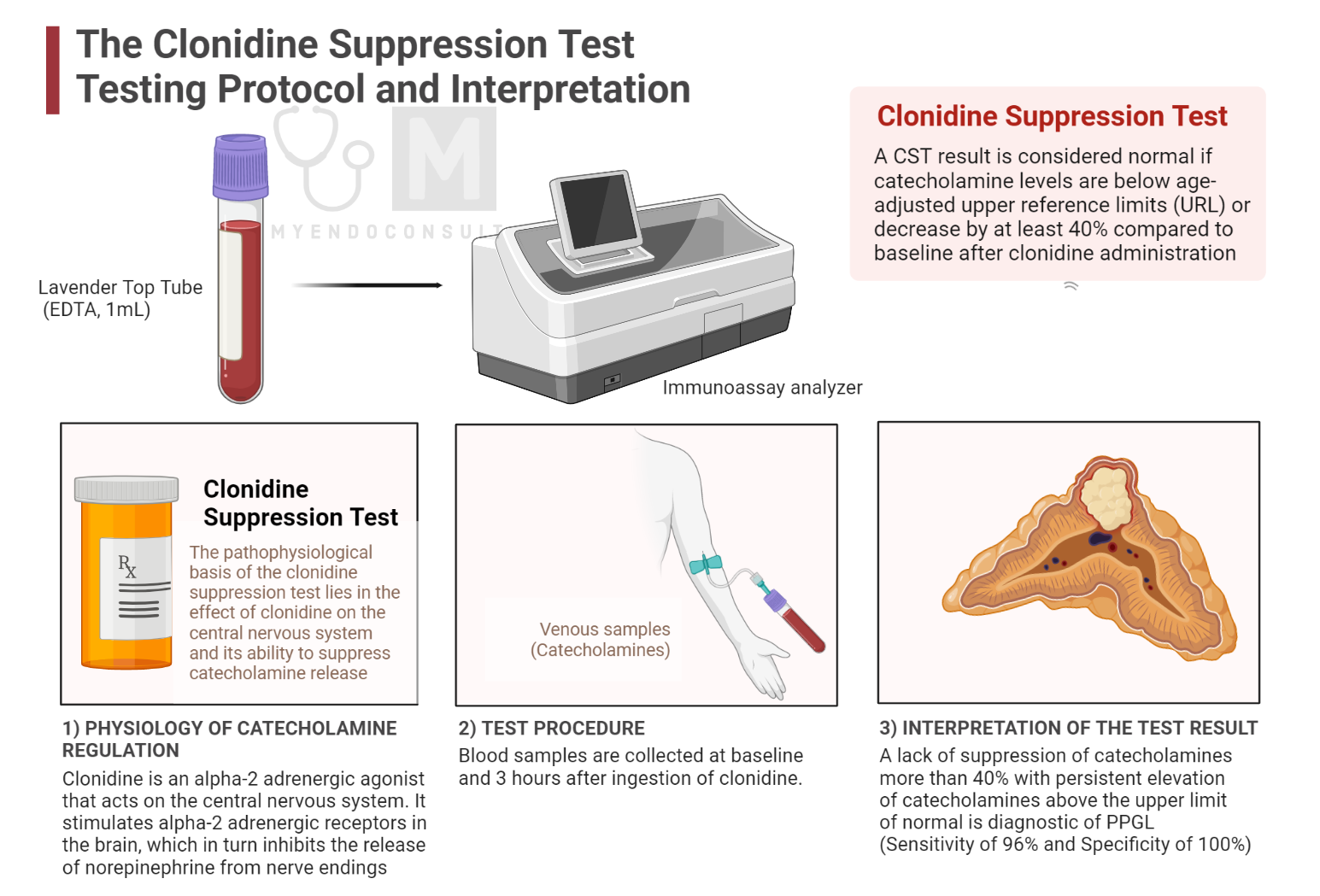
The clonidine suppression test is used to diagnose pheochromocytoma, a rare adrenal gland tumor that produces excessive amounts of catecholamines, such as epinephrine and norepinephrine. The pathophysiological basis of the clonidine suppression test lies in the effect of clonidine on the central nervous system and its ability to suppress catecholamine release[1].
Clonidine is an alpha-2 adrenergic agonist that acts on the central nervous system. It stimulates alpha-2 adrenergic receptors in the brain, which in turn inhibits the release of norepinephrine from nerve endings[2]. In a healthy individual, this leads to a decrease in circulating catecholamines (epinephrine and norepinephrine) and therefore to a reduction in blood pressure.
In the case of pheochromocytoma, tumor cells produce and secrete catecholamines independently of the control of the central nervous system. Consequently, when Clonidine is administered during the suppression test, it will not significantly suppress the release of catecholamines from the tumor, as does in a healthy individual[3].
Mechanism of action of clonidine
Clonidine can reduce norepinephrine levels in the blood of healthy individuals by activating central alpha-adrenergic receptors. As the natural increase in catecholamines relies on the activation of the sympathetic nervous system, while tumor-related release is thought to occur independently, Clonidine can help differentiate between a healthy state and a true pheochromocytoma. This is because it inhibits catecholamine release that is triggered by the nervous system but not autonomous secretion caused by pheochromocytomas[4].
Test Protocol and Practice Guide
The Clonidine Suppression Test (CST) is performed in patients between 8:00 a.m. and 9:00 a.m. Patients are instructed to avoid certain medications, smoking, and food for 12 hours prior to the test. During the test, the patients remained supine, with their blood pressure and heart rate monitored every 30 minutes. Blood samples are then collected after 30 minutes of supine rest and 3 hours after ingestion of Clonidine, with the dose adjusted according to body weight. A CST result is considered normal if catecholamine levels are below age-adjusted upper reference limits (URL) or decrease by at least 40% compared to baseline after clonidine administration[5].
Clinical Trial Evidence for the Clonidine Suppression Test
In a single-center prospective study involving 60 patients with suspected pheochromocytoma / paranglioma (PPGL), the clonidine suppression test (CST) in the diagnosis or excluding PPGL in patients with adrenal tumors and/or arterial hypertension was evaluated. Of the 46 patients who entered the final analysis, CST demonstrated 100% specificity in excluding false positive normetanephrine (NMN) results. The sensitivity of CST increased from 85% to 94% when tumors with increased isolated metanephrine (MN) were not considered. CST accurately identified all patients without PPGL who had elevated baseline NMN. The study concluded that CST is a useful diagnostic tool for differentiating elevated plasma-free NMN in patients with suspected PPGL, correctly identifying all false positive NMN screening results[5].
References
1. Remde, H., Pamporaki, C., Quinkler, M., Nölting, S., Prejbisz, A., Timmers, H.J.L.M., Masjkur, J., Fuss, C.T., Fassnacht, M., Eisenhofer, G., and Deutschbein, T. (2022) Improved Diagnostic Accuracy of Clonidine Suppression Testing Using an Age-Related Cutoff for Plasma Normetanephrine. Hypertension, 79 (6), 1257–1264.
2. Giovannitti, J.A., Thoms, S.M., and Crawford, J.J. (2015) Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesth Prog, 62 (1), 31–38.
3. Bravo, E.L., and Tagle, R. (2003) Pheochromocytoma: State-of-the-Art and Future Prospects. Endocrine Reviews, 24 (4), 539–553.
4. Bravo, E.L., Tarazi, R.C., Fouad, F.M., Vidt, D.G., and Gifford, R.W. (1981) Clonidine-Suppression Test. New England Journal of Medicine, 305 (11), 623–626.
5. Tsiomidou, S., Pamporaki, C., Geroula, A., Van Baal, L., Weber, F., Dralle, H., Schmid, K.W., Führer, D., and Unger, N. (2022) Clonidine suppression test for a reliable diagnosis of pheochromocytoma: When to use. Clinical Endocrinology, 97 (5), 541–550.
Kindly Let Us Know If This Was helpful? Thank You!


