Osteoporosis is a condition in which the bones become weak and brittle, making them more susceptible to fractures. While osteoporosis can affect people of any age, it is most commonly seen in older adults. There are a number of different treatments available for osteoporosis, and the best course of treatment will vary depending on the individual. In general, however, medical treatment for osteoporosis focuses on two main goals: increasing bone density and reducing the risk of fractures. Important clinical pearls in the management of osteoporosis will be briefly reviewed next.
Understanding Basic Osteoporosis Terminology
DEXA: Bone density should be performed in women age 65 and older and men age 70. In post-menopausal women and men age 50-69, bone density could be done if risk factors present. In patients with fracture, bone density should be performed. A significant change in bone mineral density is variable from one machine to the next and radiologist should comment if change meets statistical significance (2-3% is common).
ESTIMATION OF RISK: A fracture risk calculator is available online. Patient’s individual risk can be calculated based upon age, weight, height, bone density (at the femoral neck only), and medical history as well. This tool is helpful in estimating risk for future fracture. This tool is valid for patients who are treatment naïve. The calculator is available at http://www.shef.ac.uk/FRAX/ (Please note that the DEXA machine utilized at Sanford in Fargo and Bemidji is a GE Lunar machine and that the database used for establishing risk is variable based upon location and ethnicity.)
Nutrition Counseling
CALCIUM: Recommend that patients obtain at least 1200mg daily in total. Additional calcium may be necessary for patients with malabsorption and other disorders, and less may be necessary for patients with certain other medical conditions. High doses risk kidney stone development. It is best to get calcium from dietary sources if possible. For patients who have undergone gastric bypass or for patients on proton pump inhibitors who require supplemental calcium, calcium citrate is preferred to calcium carbonate for absorption.
VITAMIN D: Recommend vitamin D level of 30ng/dL as lower limit of acceptable values, though some physicians recommend levels between 40-60ng/dL. Typical safe daily intake is 800-1000 units cholecalciferol daily for patients 50 and older. Many patients require more than this to maintain normal levels of vitamin D. Some patients may not require any, and risks are present with high doses. All patients are different and these recommendations may or may not apply to individual patients.
Physical Activity Counseling
WEIGHT-BEARING ACTIVITY: When possible, patients should participate in weight-bearing exercise, such as walking. Physical therapy is encouraged for patients at risk of falls. Fall prevention should be emphasized along with safety of the living environment (avoiding throw rugs, electric cords, small pets, etc).
Lifestyle Counseling
All patients should be encouraged to stop smoking and to avoid excessive alcohol intake.
Pharmacology
Treatment is indicated if hip or vertebral fracture (clinical or morphometric). After a thorough history and physical examination, secondary cause evaluation of bone loss/bone health may be reasonable. Appropriate testing may include: complete blood count, chemistry group, work-up for hypogonadism, cushing’s syndrome, myeloma, vitamin D deficiency, celiac disease, thyroid dysfunction, hypophosphatasia, renal and liver dysfunction, osteomalacia, hyperparathyroidism, hypercalciuria, malabsorption, inflammation. If these are found, treating the underlying condition may significantly improve bone health. Certain medications such as steroid medications (including injections, oral, inhaled, and potentially nasal), anticonvulsants, Depo-provera, SSRI, thiazolidinediones, and proton pump inhibitors, can lead to bone loss.
If there are any questions about which medication to choose, or how to proceed with the secondary cause evaluation, or to obtain more patient-specific recommendations, an endocrinology consultation is welcome. The use of an expensive medication, such as teriparatide, is often worth evaluation for secondary causes to be sure the medication can be efficacious. There are many medications available to decrease fracture risk. The effects on bone density and fracture risk reduction are as below.
| Bone Density Effects (spine) | Bone density Effects (hip) | Spine fracture risk reduction | Hip fracture risk reduction | Nonvertebral fracture risk reduction | |
| Calcitonin (Miacalcin) | None | None | |||
| Raloxifene (Evista) | ++ | + | - | No data | No data |
| Oral bisphosphonates (Alendronate, Risedronate, Ibandronate) | +++ | ++ | - | - | - |
| IV bisphosphonates (Ibandronate, Zoledronic acid) | +++ | ++ | - | - | - |
| Denosumab (Prolia) | + | + | - | - | - |
| Teriperitide (Forteo) | ++++ | + | - | No data | - |
Calcitonin (Miacalcin)
Mechanism of Action: directly inhibit osteoclast bone resorption
FDA approved Indications: Postmenopausal osteoporosis, hypercalcemia, Paget’s disease
Contraindication: Hypersensitivity to calcitonin
Precaution: Could cause hypocalcemic tetany
Side Effects: Flushing, nausea, dizziness (orthostasis), allergic reaction, injection site reaction, rash, nasal irritation, peripheral edema, eye pain, visual changes, increased urination
Monitoring: Bone density, nasal examination, monitor for allergy, bone turnover markers, evidence for fracture, urine sediment
Raloxifene (Evista)
Mechanism of Action: Selective Estrogen Receptor Modulator, reducing bone resorption and increases BMD (bone mineral density)
FDA approved Indications: Post-menopausal osteoporosis—treatment and prophylaxis; Invasive breast cancer with postmenopausal osteoporosis, prophylaxis; Invasive breast cancer with postmenopausal women at high risk, prophylaxis
Contraindication: VTE history, nursing mothers, pregnant women or women who may become pregnant
Black box warning: Increased risk for DVT and PE. Women with active clotting or history of VTE should not use raloxifene. Increased risk of death due to stroke in post-menopausal women with history of CAD or at risk for cardiac event who took raloxifene was seen. Consider risk/benefit ratio in women at risk for stroke
Precaution: immobilization period, thromboembolic disease or risk of, CAD or high cardiac risk, hypertriglyceridemia, premenopausal women, use with estrogen, stroke risk.
Side Effects: Hot flashes, peripheral edema, clotting, hypertriglyceridemia, retinal vein thrombosis
Monitoring: breast exams, mammograms, bone density, monitor for adverse effects especially in patients with hepatic or renal dysfunction, eye exams, INR in patients on Coumadin starting or stopping therapy triglycerides
Oral bisphosphonates
Mechanism of Action: binds to bone hydroxyapatite, decreases osteoclast activity, affects bone metabolism
FDA approved Indications: Alendronate (Fosamax): male osteoporosis, corticosteroid-induced osteoporosis, Paget’s disease, postmenopausal osteoporosis, prophylaxis for post-menopausal osteoporosis. Risedronate (Actonel): male osteoporosis, corticosteroid-induced osteoporosis, prophylaxis for corticosteroid-induced osteoporosis, Paget’s disease, postmenopausal osteoporosis, prophylaxis for post-menopausal osteoporosis. Ibandronate (Boniva): postmenopausal osteoporosis, prophylaxis for post-menopausal osteoporosis
Contraindications: esophageal dysmotility, uncorrected hypocalcemia, inability to sit/stand for 60minutes, hypersensitivity to the medication
Precaution: renal dysfunction, hypocalcemia, hypovitaminosis D, atypical fractures of the thigh, Barret’s esophagus, esophagitis, ulcers, upper GI irritation, bone pain, renal impairment (CrCl <30), concurrent use with proton pump inhibitors. Osteonecrosis of the jaw—increased risk with anemia, coagulopathy, cancer patients, steroid use, chemotherapy, radiation, dental disease, infection, dental procedures
Side Effects: Ulceration of esophagus, duodenum, gastric; arthralgia, osteonecrosis of the jaw, bone pain, myalgia, GI symptoms, flu-like symptoms, fever, vomiting, rash, asthenia
Monitoring: Paget’s disease –improvement in signs/symptoms, normalization of alkaline phosphatase . Osteoporosis—BMD, decreased fracture incidence. Glucocorticoid-induced—monitor BMD. BMD, Dental exams for signs of osteonecrosis of the jaw, biochemical markers of bone formation/resorption, evidence of fractures, calcium, electrolytes, phosphate levels, renal function, joint/bone/muscle pain, signs of esophageal reaction/dysphagia, disorders affecting bone metabolism (calcium levels)
IV bisphosphonates
Mechanism of action: (Zoledronic Acid) inhibits bone resorption, inhibits osteoclast activity, induces osteoclast apoptosis, blocks resorption of mineralized bone and cartilage through bone binding.
FDA approved indications: Zoledronic acid (Reclast) : postmenopausal osteoporosis, male osteoporosis, prophylaxis for post-menopausal osteoporosis, secondary osteoporosis prophylaxis in patients with low trauma hip fracture, corticosteroid-induced osteoporosis prophylaxis or treatment, Paget’s disease, hypercalcemia, multiple myeloma, bone metastasis. Ibandronate (Boniva): postmenopausal osteoporosis, postmenopausal osteoporosis prophylaxis
Contraindications: CrCl <35, hypocalcemia, renal failure, hypersensitivity to the medication
Precaution: renal dysfunction, hypocalcemia, hypovitaminosis D, atypical fractures of the thigh, aspirin-sensitive asthma, bone pain, renal impairment (CrCl <30), pregnancy (should not use). Osteonecrosis of the jaw—increased risk with anemia, coagulopathy, cancer patients, steroid use, chemotherapy, radiation, dental disease, infection, dental procedures
Side Effects: Flu-like symptoms; arthralgia, osteonecrosis of the jaw, bone pain, myalgia, fever, vomiting, renal failure, atrial fibrillation, CVA, dyspnea
Monitoring: calcium, alkaline phosphatase in Paget’s disease, skeletal events, BMD, creatinine, calcium, phosphorus, magnesium (especially in those at risk for hypocalcemia), fluid status (should be hydrated prior to use)
Denosumab (Prolia)
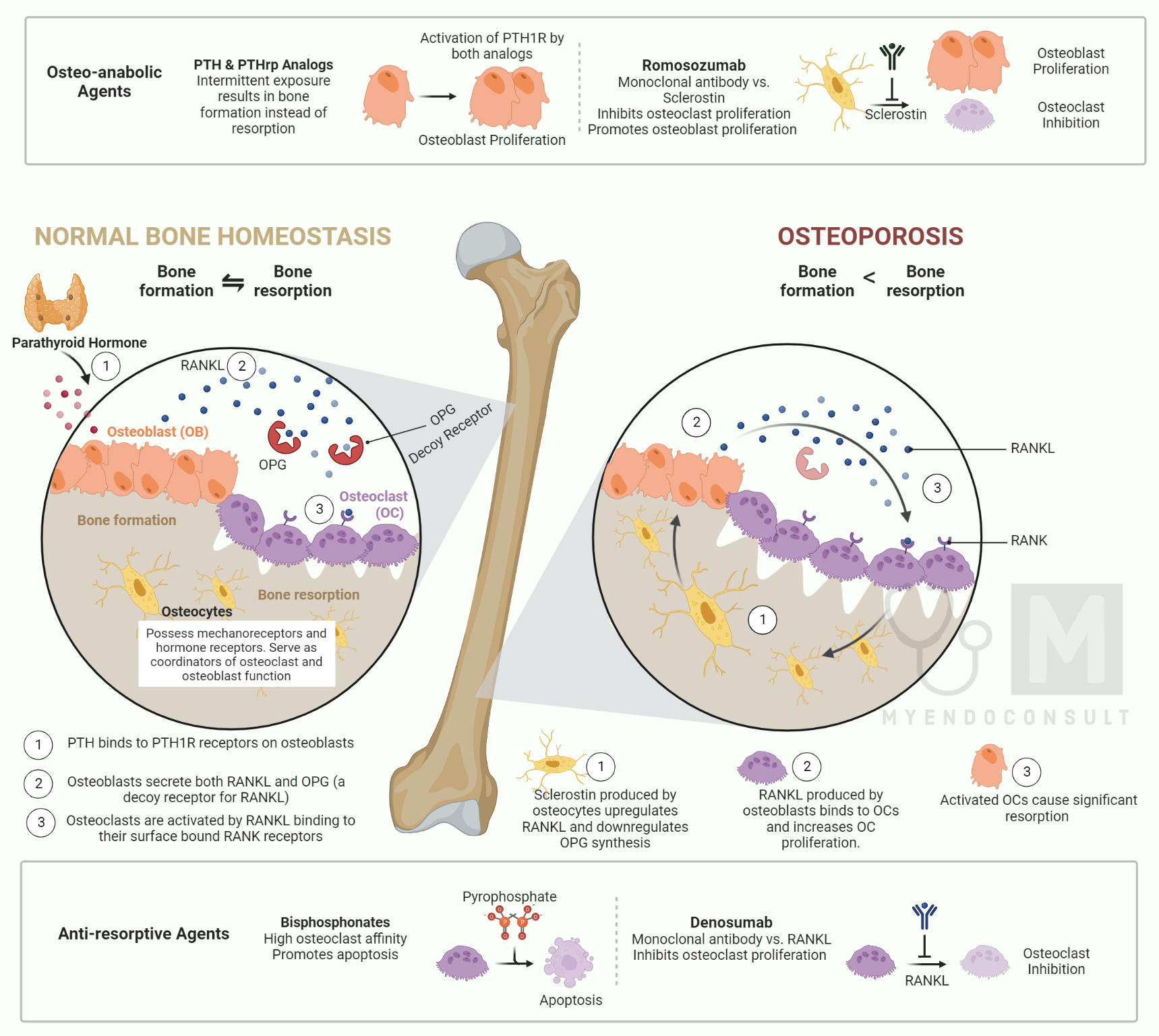
Mechanism of Action: receptor activator of NF Kappa B Ligand (RANKL) inhibitor resulting in decreased osteoclast activity and decreased bone resorption
FDA approved indications: postmenopausal osteoporosis, osteopenia in men at high risk receiving androgen-deprivation therapy for non-metastatic prostate cancer, breast cancer in women with osteopenia receiving aromatase therapy, bone metastasis with solid tumors
Contraindications: hypocalcemia
Precautions: hypocalcemia, especially in renal failure patients (monitoring necessary per nephrology recommendations/protocol), at risk for osteonecrosis of the jaw (as above)
Side Effects: infections, hypocalcemia, hypophosphatemia, rash, diarrhea, arthralgia, fatigue
Monitoring: skeletal events/fractures, calcium, phosphorus, magnesium, dental exams for osteonecrosis of the jaw, signs and symptoms of bone turnover suppression such as atypical fractures and delayed fracture healing.
Teriparatide (Forteo)
Mechanism of Action: works like PTH to activate osteoblasts when given in a pulsatile fashion
FDA approved indications: osteoporosis (primary or hypogonadal), osteoporosis due to corticosteroids, postmenopausal osteoporosis
BLACK BOX WARNING: osteosarcoma has been reported in laboratory animals, so it should not be used in patients with unexplained elevation in alkaline phosphatase, history of implanted or external beam radiotherapy, Paget’s disease, open epiphyses
Contraindications: hypersensitivity to the medication
Precautions: active or recurrent kidney stones, bone metastasis/skeletal malignancies, metabolic bone disease, Paget’s disease, open epiphyses, patients at risk for osteosarcoma, pediatric populations, history of radiation, preexisting hypercalcemia, unexplained elevation of alkaline phosphatase, use for more than 2 years not recommended, orthostasis has been reported
Side effects: angina, hypotension, syncope, nausea/vomiting, arthralgia
Monitoring: biochemical markers of bone formation/resorption, fracture, calcium, urine calcium in patients with history of nephrolithiasis/hypercalciuria, uric acid, orthostatic hypotension, signs/symptoms of osteosarcoma
References
- Recommendations adapted from National Osteoporosis Foundation recommendations. http://www.nof.org/
Kindly Let Us Know If This Was helpful? Thank You!


