Cushing’s syndrome is a disorder of chronic glucocorticoid excess and is characterized by features of protein catabolism and varying signs and symptoms. The most common cause of Cushing’s syndrome is the exogenous administration of glucocorticoids.
However, other causes of Cushing’s syndrome include ACTH-secreting pituitary adenomas (Cushing’s disease accounts for approximately 90% of cases), ectopic ACTH production, and adrenal abnormalities. Patients with Cushing’s syndrome typically present with weight gain, facial rounding (moon face), easy bruising, and hypertension.
Furthermore, patients may experience osteoporosis, insulin resistance, and psychological changes such as depression and anxiety. Diagnosis of Cushing’s syndrome can be difficult as the signs and symptoms are nonspecific. However, available diagnostic tests including urinary-free cortisol excretion, late-night salivary cortisol levels, and the dexamethasone suppression test can help to confirm the diagnosis.
Once the diagnosis of Cushing’s syndrome has been established, treatment typically involves surgery, radiation therapy, or medical therapy. In cases where the underlying cause is exogenous administration of glucocorticoids, discontinuation of the offending medication typically resolves symptoms.
Pathophysiology of Cushing’s syndrome
Cushing’s syndrome is a condition that occurs when the body is exposed to high levels of the hormone cortisol. Cortisol is produced by the adrenal glands and plays an important role in regulating metabolism, immune function, and blood pressure. However, when cortisol levels become too high, it can lead to several health problems, including weight gain, hypertension, diabetes, and osteoporosis. Cushing’s syndrome can be caused by a tumor on the adrenal gland or by taking certain medications (such as corticosteroids).
| Subtype of Cushings syndrome | Clinical features |
| Pseudo-Cushings syndrome | These are reversible conditions that can present with sins of Cushings syndrome, elevated serum cortisol levels and even abnormal dexamethasone suppression tests. Examples include depression, morbid obesity, uncontrolled diabetes mellitus, PCOS and metabolic syndrome |
| Cyclical Cushings syndrome | Cortisol levels fluctuate in a cyclical pattern, with peak levels occurring every few weeks or months. During the peak phase, patients may experience weight gain, fatigue, and mood swings. The trough phase is when cortisol levels are at their lowest and patients may feel less symptomatic. Defined as the presence of three peaks and two troughs of cortisol secretion over a period of time. |
| Subclinical Cushings syndrome | Lack of specific symptoms of hypercortisolemia, but with biochemical evidence of autonomous cortisol production. Example, following a 1mg DST 0800h cortisol levels are typically above 5mcg/dL (100% specificity) compared to the cutoff for overt Cushing’s syndrome (i.e., >1.8mcg/dL). Low DHEAs and ACTH at baseline. |
| Pituitary Cushings syndrome (ACTH-dependent) | Common in young females with a gradual onset. ACTH mediated hyperpigmentation of palmar creases (in non-blacks), mucosal surfaces, scar tissue). Present with virilization (ACTH mediated production of adrenal androgens) |
| Exogenous Cushings syndrome (ACTH-independent) | History of exposure to supraphysiologic exogenous glucocorticoids. ACTH is suppressed as such, they lack hyperpigmentation and virilization (ACTH mediated production of adrenal androgens) |
| Ectopic Cushings syndrome (ACTH-dependent) | Occurs in cases of ectopic ACTH secretion such as bronchial carcinoids. Present with features of pituitary Cushings syndrome, but additional may have features of neuroendocrine tumors (flushing, diarrhea and brochospasm) |
| Adrenocortical carcinoma (ACTH-independent) | Rapid onset of clinical features of hypercortisolemia. Classically have worsening or new onset hypertension and virilization. ACTH is suppressed as such, they lack hyperpigmentation. |
Clinical Features
The most common symptoms include moon facies (a round, full face), buffalo hump (a fatty deposit on the upper back), violaceous striae (broad purple streaks on the skin of a fair-skinned patient), myopathy (weakness and wasting of muscle tissue), and thin skin. Cushing syndrome can also lead to hypertension, osteoporosis, diabetes, and mood changes in some cases. All of these findings are considered to be positive discriminatory findings of Cushing syndrome – facial plethora, violaceous striae, myopathy, and thin skin.
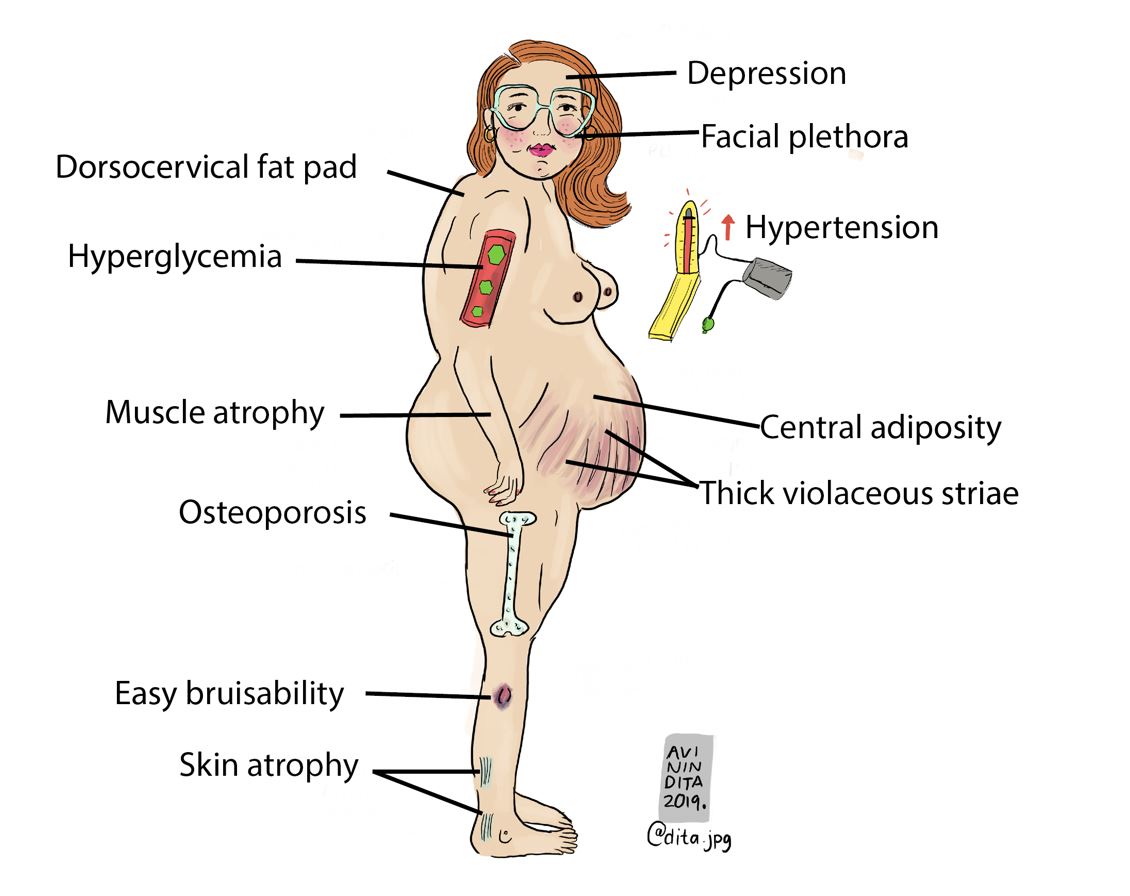
Investigations
Tests to establish the diagnosis of Cushing’s syndrome
– 24-hour urinary cortisol
– Late-night salivary cortisol (2 consecutive samples on separate nights)
– Low dose dexamethasone suppression test
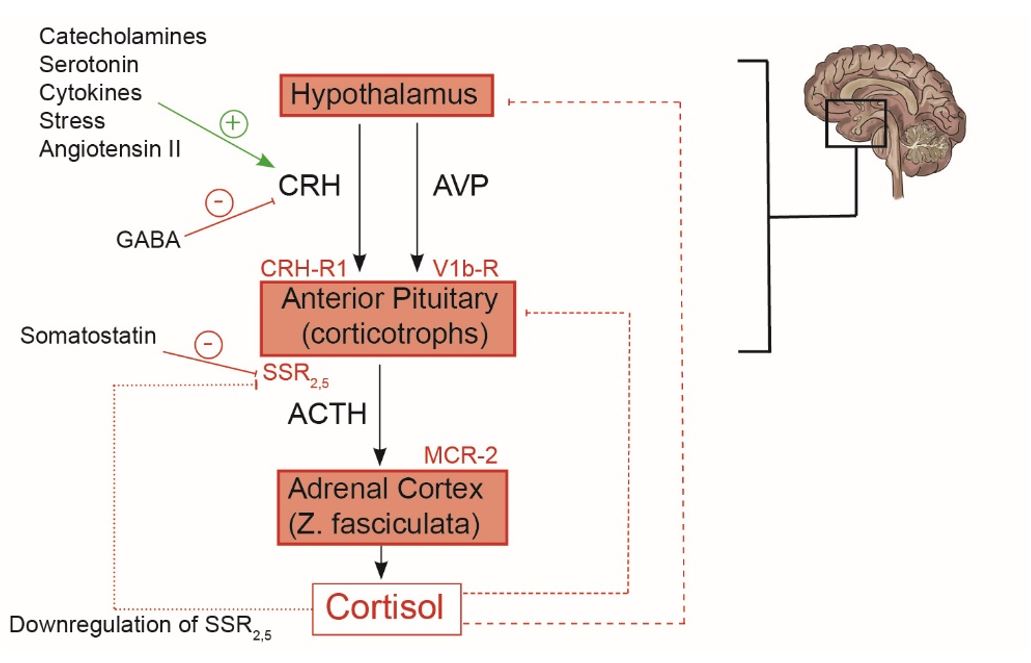
Regulation of serum cortisol
Cortisol is a stress hormone released in response to physical or psychological stress. Cortisol is released by the adrenal gland in response to signals from the brain (i.e., CRH and ACTH). Cortisol levels are highest in the morning (around 8 am) and decline throughout the day. There is a loss of this diurnal rhythm of cortisol secretion in patients with Cushing’s syndrome.
Dynamic Tests for Cushing’s syndrome
The use of dexamethasone in evaluating Cushing’s syndrome was first described in detail by Dr. Grant Liddle in 1960. He was able to show that dexamethasone administered at a dose of 0.5mg every six hours for 8 doses induced complete suppression of adrenal cortisol synthesis in normal people. In contrast, patients with Cushing’s syndrome were unable to suppress adrenal cortisol production.
The original Liddle’s protocol involved the measurement of urinary 17-hydroxycorticoid excretion after administration of dexamethasone. The measurement of urinary steroids has a low sensitivity and specificity; as such, serum cortisol levels are now utilized in dynamic tests.
Dexamethasone is the most potent glucocorticoid in suppressing the hypothalamo–pituitary-adrenal axis (17 times more potent than hydrocortisone). Dexamethasone easily crosses the blood-brain barrier as it does not bind to cortisol-binding globulin (CBG). In addition, dexamethasone has no cross-reactivity with hydrocortisone and other glucocorticoid metabolites; therefore, it does not interfere with the cortisol assay.
More importantly, dexamethasone’s binding affinity to the glucocorticoid receptor (GR) is 50% greater than that of cortisol with an anti-inflammatory potency approximately 25 times that of prednisolone. When administered orally, dexamethasone has a bioavailability of almost 100%.
Parenteral administration results in similar bioavailability but with peak plasma concentrations achieved 2-6 hours later than with oral administration. Dexamethasone has a relatively long half-life (t ½) in plasma (approximately 2.5-4 hours) and is slowly eliminated from the body. The HPA axis typically resets within 24hrs after a single administration of dexamethasone (1mg).

Protocol for the low dose Dexamethasone Suppression Test
Administer 1mg of dexamethasone (orally) between 11 pm, and 12 am. Dexamethasone will reach peak plasma concentrations approximately four hours after oral administration. This will inhibit the physiological secretion of ACTH, which is expected to start around 3 am and peak around 7 am.

Interpretation of early morning cortisol sampled around 8 to 9 am. The threshold for diagnosing endogenous hypercortisolemia is >1.8mcg/dL (50 nmol/L). This results in a sensitivity and specificity of 95% and 80%, respectively. By raising the diagnostic threshold to 5mcg/dL (140 nmol/L) specificity improves to greater than 95%.
This, unfortunately will lead to a decline in sensitivity (trade-off of sensitivity for specificity). DST is a screening test; as such, it should be highly sensitive. This explains the use of the 1.8mcg/dL diagnostic threshold as a means of diagnosing endogenous hypercortisolemia. Learn more about the causes of false positive and false negative dexamethasone suppression tests.
https://youtu.be/P22rjlgzT7Q
Inferior Petrosal Sinus Sampling
Inferior petrosal sinus sampling (IPSS) is a diagnostic procedure used to identify the source of excess cortisol in patients with Cushing’s syndrome. The procedure involves the placement of a catheter in the inferior petrosal sinus, a drainage vein that drains blood from the pituitary gland. Cortisol levels are then measured in the blood before and after the administration of corticotropin-releasing hormone (CRH). If the IPSS reveals that the source of excess cortisol is the pituitary gland, this is called “lateralization.” The procedure is considered to be highly accurate, and it is often used as a first-line diagnostic tool in cases of suspected Cushing’s syndrome.
Surgical Treatment
Surgery is the most effective treatment for Cushing’s syndrome. The most common type of surgery is transsphenoidal surgery, which involves removing the portion of the pituitary gland that is producing excess cortisol. In some cases, bilateral adrenalectomy may also be necessary in order to completely remove all sources of excess cortisol from the body.
What is Nelson’s syndrome?
Nelson’s Syndrome is a rare condition that occurs when there’s an increase in CRH production after total bilateral adrenalectomy for patients with pituitary Cushing’s syndrome, regardless of previous surgery. The increased levels of CRH promote the growth of a corticotropinoma because it isn’t suppressed within the hypothalamus anymore in the absence of cortisol. Consequently, corticotropinomas increase in size leading to symptoms related to mass effects of an expanding pituitary tumor.
Medical Treatment
Somatostatin analogs are drugs that mimic the action of somatostatin, a hormone that inhibits the release of other hormones, including cortisol. The most commonly used somatostatin analog is pasireotide (Signifor), which is effective in lowering cortisol levels and improving symptoms.
Retinoic acid (RA) is a medical treatment for Cushing’s syndrome. RA works by inhibiting the production of COUP-TF1, a protein that is essential for cortisol synthesis. As a result, RA can help to reduce the levels of cortisol in the body and improve the symptoms of Cushing’s syndrome. While RA is not a cure for Cushing’s syndrome, it can help to manage the condition and improve the quality of life for patients.
Steroidogenesis inhibitors are a class of drugs that are used to treat Cushing’s syndrome by reducing the production of cortisol. The most common agents in this class are ketoconazole, metyrapone, and mitotane. These drugs work by inhibiting the enzymes involved in cortisol synthesis in the adrenal gland. While they are effective at reducing cortisol levels, they can also cause significant side effects, such as gastrointestinal distress, adrenal insufficiency, and hepatotoxicity.
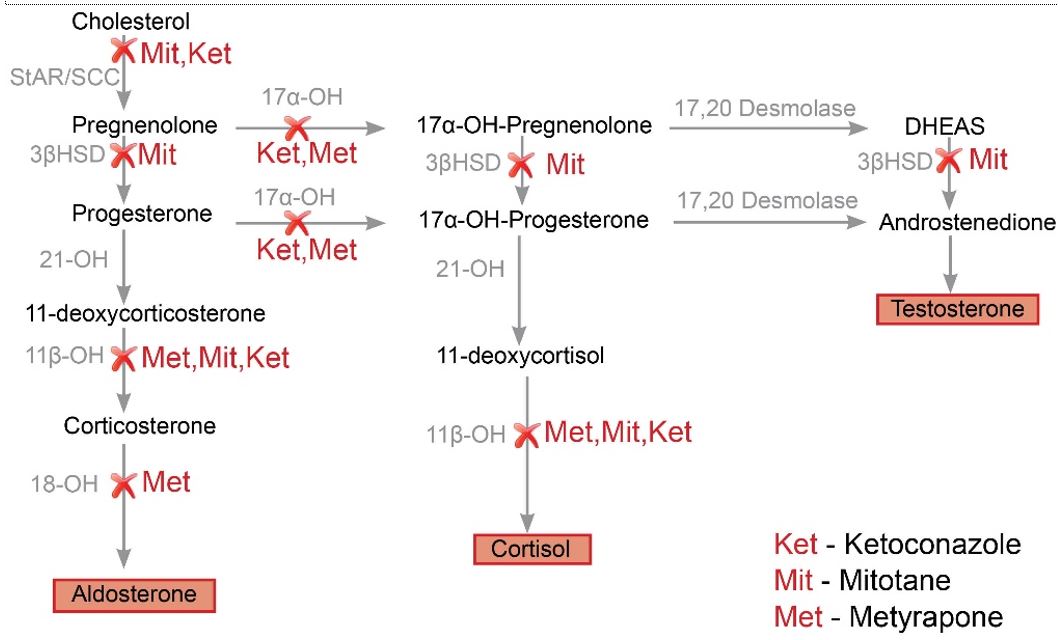
Mifepristone (RU-486) is a synthetic steroid with antiprogestational and antiglucocorticoid activity. It is a competitive inhibitor of progesterone and cortisol at the glucocorticoid receptor, and thus prevents the maturation of the corpus luteum, suppresses ovulation, and antagonizes the action of endogenous cortisol.
Screening for Cushings Syndrome in patients with comorbidities
| Pregnancy or women on contraceptives | The use of Urinary Free Cortisol (UFC) and against the use of dexamethasone testing in the initial evaluation of pregnant women |
| Seizure Disorder | recommend against the use of dexamethasone testing in patients receiving antiepileptic drugs known to enhance dexamethasone clearance and recommend instead measurements of nonsuppressed cortisol in the blood, saliva, or urine |
| Renal failure | 1-mg overnight Dexamethasone Suppression Test (DST) rather than UFC for initial testing for Cushing’s syndrome in patients with severe renal failure |
| Cyclic Cushings syndrome | UFC or midnight salivary cortisol tests rather than DSTs |
| Adrenal incidentaloma | 1-mg DST or late-night cortisol test, rather than UFC |
References
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008 May;93(5):1526-40.
- Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1960 Dec;20:1539-60.
Images(s) Courtesy
MyEndoConsult
Kindly Let Us Know If This Was helpful? Thank You!


