
In this article, the embryology, histology and gross anatomy of the adrenal glands will be reviewed.
Historical timeline of discoveries in adrenal gland anatomy
- 1563 Bartholomaeus Eustachius Sanctoseverinatus describes the adrenals and recognizes them as an organ in his treatise “De glandulis quae renibus incumbunt”.
- 1805 Cuvier differentiated medulla and cortex of the adrenal gland
- 1855 Thomas Addison gives the classic description of adrenal insufficiency and of the disease which bears his name in his book “On the constitutional and local effects of disease of the suprarenal capsules
- 1856 Brown-Sequard discovers in animal experiments that the adrenals are necessary to life.
- 1868 Frankel described the case of an adrenal tumor with pressor crises.
- 1892 Berdez discovered a chromaffin tumor of the adrenal.
- 1894 Two groups, Oliver and Schafer and Syzmonowicz and Cybulski, independently discovered the existence of pressor substances in adrenal extracts.
- 1896 Sir William Osler recognizes the clinical utility of orally administered adrenal extracts in Addison’s disease.
- 1898-1905 Forth and Abel, Takamine and Aldrich, and Stolz and Dakin isolated adrenaline, established its structural formula and synthesized it.
- 1922 First detailed clinical report of a pheochromocytoma by Labbe, Tinelle, and Doumer.
- 1926 Smith demonstrates that hypophysectomy leads to atrophy of the adrenals and Evans succeeds in preventing this atrophy by the administration of pituitary extracts.
- 1927 First successful surgical removal of a pheochromocytoma by Mayo.
- 1928-1930 effective adrenal extracts are made by Rogoff and Stewart, Hartmann and Mcarthur, Pfiffner and Swingle.
- 1932 Cushing describes the syndrome of pituitary-adrenal hyperactivity.
- 1933 Loeb discovers disturbances of serum electrolytes in Addison’s disease and bases the treatment with sodium chloride on these discoveries.
- 1937-1952 Isolation, elucidation of the constitution,and synthesis of the adrenal hormones corticosterone, deoxycorticosterone, cortisone and cortisol by Reichstein, Kendall, Wintersteiner and collaborators.
- 1942 Li and Sayers isolate ACTH.
- 1945 Holtz, Credner, and Kronenberg discovered noradrenaline.
- 1946 Sel Ye describes the general adaptation syndrome.
- 1948 Hench and collaborators detect the antiinflammatory effect of cortisone.
- 1953-1955 Isolation and elucidation of the constitution of aldosterone by Simpson and Tait, Wettstein and Neher, Reichstein and Von Euw. Synthesis of aldosterone by Wettstein and Schmidlin.
- 1954 Conn describes primary hyperaldosteronism.
- 1957 Armstrong detected vanilmandelic acid as a metabolite of catecholamines.
- 1957 Axelrod described the inactivation of catecholamines by O-methylation.
- 1958 Gross suggests the control of aldosterone secretion by angiotensin.
- 1960 Davis and Genest confirm the regulation of aldosterone secretion by angiotensin II.
- 1962 Hofmann, Li, and Schwyzer describe the amino-acid sequence of ACTH.
- 1966 Schwyzer and Sieber succeed in synthesizing beta-corticotropin.
Embryology, Gross Anatomy, and Histology of the Adrenal Cortex
Embryology: The two main components of the adrenal (suprarenal) gland, the cortex, and the medulla, differ not only in morphology and function but also in origin. The cortex develops from cells of the coelomic epithelium and is, therefore, of mesodermal origin, while the chromaffin and sympathetic ganglion cells of the medulla are derived from the neural ectoderm.
In mammals, the cortex surrounds the medulla like a capsule. In the four-week-old human embryo, coelomic epithelial (mesothelial) cells situated on both sides between the root of the mesentery and the gonadal anlagen begin to proliferate and invade the underlying mesenchymal tissue.
Here, they differentiate into compact, acidophilic epithelial masses which lose contact with the coelomic epithelium and form the primitive or fetal cortex of the suprarenal glands. During the 6th embryonic week, neural ectodermal elements, the pheochromoblasts, start to invade the primitive cortex (also see the section on “Embryology and Histology of the adrenal medulla” below).
Soon afterward, they can be observed singly or in small groups between the cortical cells. Early in the third month, the coelomic epithelium proliferates again and a new wave of mesothelial cells is shed into the underlying mesenchyma. These cells, which are smaller than those of the first wave, surround the fetal cortex to form the definitive cortex of the adrenal gland.
During the last month of intrauterine life, the fetal cortex begins to regress. At the time of birth, the suprarenal glands (adrenal glands) are nevertheless very large; their volume is approximately one-third that of the kidneys.
In the newborn, the atrophy of the fetal cortex proceeds rapidly, except for its outermost cell layers, which are taken up into the zona reticularis of the definitive cortex. In the adult human, the size of the suprarenal glands is only about 1/30 of that of the kidneys. The function of the primitive suprarenal cortex is still poorly understood.
Since the adrenal glands are atrophic in anencephalic infants (in whom the hypophysis is absent), it is assumed that the very large size of the fetal glands is due to the release of adrenocorticotrophic hormone (ACTH) from the fetal pituitary. ACTH secretion by the fetus can also be inhibited by cortisone administration to the mother. In such cases, the fetal suprarenal gland is often found to be hypoplastic or even atrophic.
Gross Anatomy: In the adult, each of the adrenal glands weighs about 3-5 g. They are situated retro-peritoneally and on the superomedial aspect of the anterior aspect of the kidneys. Both glands are surrounded by adipose tissue and by the renal fasciae, to which they adhere firmly.
Unlike the kidneys, the glands are not displaced during respiration or changes in posture. The right adrenal gland is pyramidal in shape. Its base rests on the kidney, and its medial portion projects to some extent behind the inferior vena cava. Behind, it lies against the diaphragm, whereas in front, it is in direct contact with the right lobe of the liver, with the dorsal wall of the vena cava, and with the peritoneum.
The left suprarenal gland is usually semilunar in shape and more flattened. In front, it is in close contact with the splenic artery, with the peritoneum of the lesser sac, and with the pancreas. Its dorsal portions rest on the diaphragm.
The phrenic artery supplies the adrenal gland with multiple branches, the superior adrenal arteries. The middle suprarenal artery reaches the gland directly from the aorta, whereas the inferior adrenal arteries emerge from the renal artery. The arrangement of these blood vessels varies widely not only in different people but also on the two sides of the body. The suprarenal vein leaves the gland in the hilus. In addition, some small veins accompany the arteries.
The left adrenal vein enters the renal vein, whereas the right vein drains into the inferior vena cava. A number of lymphatic vessels arise in the adrenal medulla.
They follow the veins and supply adjacent lymph nodes. In contrast, the cortex contains only a very few lymphatics. The predominantly preganglionic, sympathetic nerve fibers of the adrenal glands arise in the celiac plexus or are branches of the thoracic and lumbar splanchnic nerves.
Most of these fibers supply the specific medullary cells; a few of them, however, contact the ganglion cells in the organ capsule and in the medulla. Postganglionic nerve fibers extend to the blood vessel walls. No parasympathetic fibers seem to enter the glands.
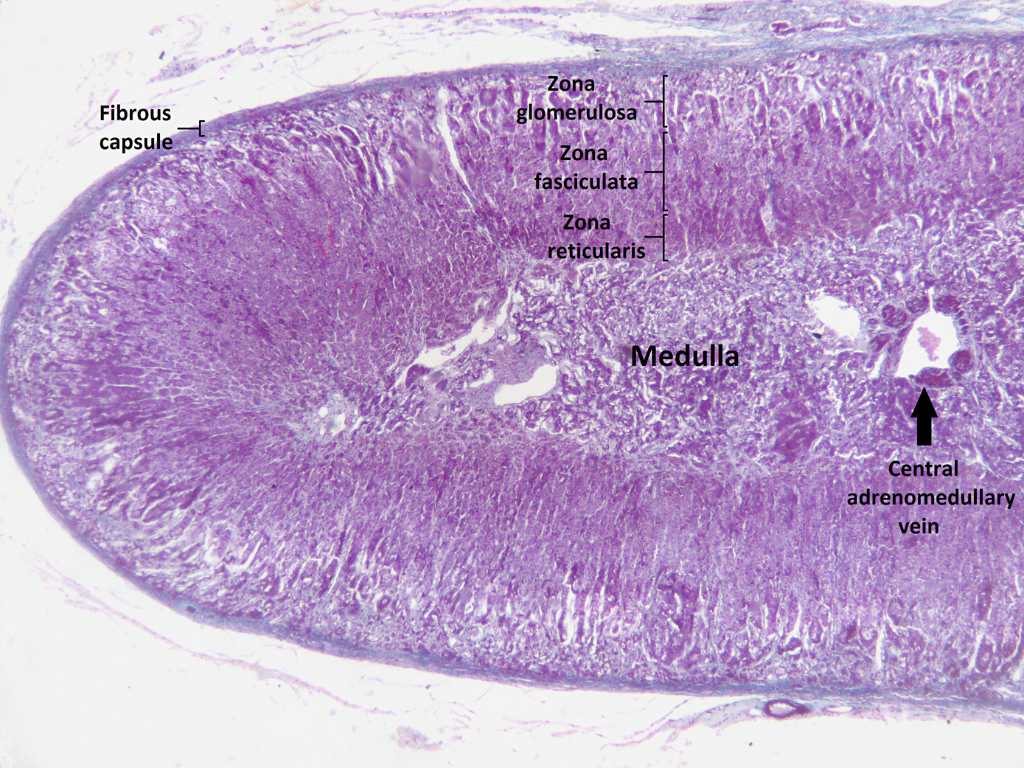
Histology: A thick capsule of collagenous connective tissue surrounds the suprarenal gland and extends as trabeculae into the cortex. It contains an extensive arterial plexus which is supplied by the various suprarenal arteries. The cortical arterioles arising from this plexus branch into the sinusoidal capillaries, which surround the cords and groups of cortical parenchymal cells.
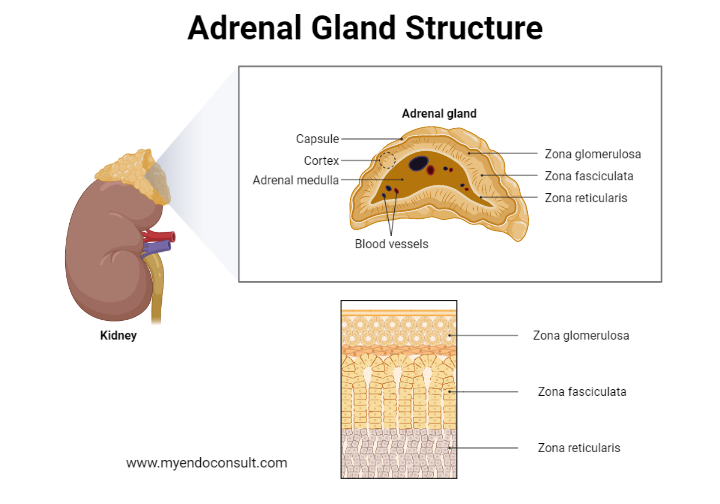
In addition, the capsule and its trabeculae display some lymphatic vessels as well as a nerve plexus. The latter includes a large number of mainly preganglionic fibers and a few sympathetic ganglion cells. Its branches penetrate the cortex in the trabeculae and terminate in the medulla.
A framework of reticular fibers extends between the trabeculae, the organ capsule, and the blood vessels. It supports the specific cortical cells as well as the medullary cells and surrounds the capillary network of the organ.
The adrenal cortex constitutes approximately 80% of the total organ volume and is composed of three easily distinguishable concentric zones.
The thin zona glomerulosa adjacent to the organ capsule consists of groups of rather small, epitheloid cells which contain a deeply staining nucleus displaying one or two nucleoli. The cytoplasm is acidophilic and contains some small clumps of basophilic material.
The small lipid droplets and the elongated mitochondria are randomly distributed throughout the cytoplasmic matrix, whereas the Golgi apparatus is situated in a juxtanuclear position. Both reticular fibers and sinusoidal capillaries surround the cell groups. Electron-microscopically, the zona glomerulosa cells are characterized by an anastomosing network of smooth-surfaced endoplasmic reticulum extending all over the cell body.
Only a few profiles of granular endoplasmic reticulum can usually be observed. The mitochondria possess lamellar cristae thus differing from those of the other cortical zones. The cytoplasmic matrix contains large numbers of polyribosomes and the lipid droplets are often arranged in small groups. The Golgi apparatus does not differ from that of the cells of other organs.
The cell columns of the largest cortical zone, the zona fasciculata, are continuous with the cell groups of the zona glomerulosa. Their polygonal cells form long cords which are usually one to two cells thick and are arranged radially to the organ capsule. The individual columns are separated by reticular fibers and by the sinusoidal capillaries. The fasciculata cells often contain two nuclei, and their cytoplasm is characterized by the presence oflarge numbers of lipid droplets.
In routine histological sections, the cytoplasm appears highly vacuolated. When observed under the electron microscope, the cells of this zone display an extensive network of agranular endoplasmic reticulum. The rounded mitochondria vary considerably in size but are usually much larger than those of the other cortical zones. Their numerous cristae appear as vesicular invaginations of the inner mitochondrial membrane or as vesicles lying free in the mitochondrial matrix. The lipid droplets are dispersed throughout the cytoplasmic matrix.
The zona reticularis adjacent to the adrenal medulla consists of an anastomosing network of epitheloid cells which vary considerably in size and shape. Within the cell groups, “light” and “dark” cells can be distinguished, the latter presumably being degenerative elements, since they contain hyperchromatic and shrunken nuclei and large masses of lipofuscin pigment.
Compared with the fasciculata cells, the light cells of the zona reticularis contain a much smaller number of lipid droplets. The shape of the mitochondria and the amount of agranular endoplasmic reticulum, however, do not differ significantly. The surface of the cortical cells of the three zones is usually enlarged by small infoldings as well as by numerous small microvilli which project into the intercellular and perivascular spaces.
The endothelium of the sinusoidal capillaries is, over large regions, extremely attenuated and fenestrated. The endothelial fenestrae or pores are closed only by a very thin diaphragm which separates the blood from the basal lamina surrounding the sinusoids. A few small bundles of collagen fibrils are dispersed in the perivascular spaces. They correspond to the reticular fibers revealed by light microscopy. Next, we will discuss the anatomy of the adrenal medulla.
Embryology and Histology of the adrenal medulla
Embryology : In the human embryo about 11-12 mm in length (early 6th week), neuroblasts which have separated from the primitive ganglia of the celiac plexus come into contact with the medial portions of the adrenocortical primordium. At the end of the 6th week, (embryonic length about 14 mm), these sympathetic cells begin to invade the primitive adrenal cortex.
As early as the 12th week, they are found to contain small amounts of norepinephrine, and at about 22 weeks the chromaffin reaction becomes positive. By this time, most of the sympathetic neuroblasts have accumulated in the central portions of the developing gland where they become grouped in cords and clusters of varying size.
Migration of neuroectodermal elements into the fetal adrenal gland continues, however, throughout the prenatal period. The majority of them differentiate into specific medullary cells; a small number, however, develop into sympathetic ganglion cells, which are scattered singly or in small groups throughout the adrenal medulla.
Histology : The medullary tissue is usually not well preserved in routine necropsy material. When it has been fixed under favorable conditions, however, it is found to be composed of polymorphic epitheloid cells with a clear, rounded nucleus and a large number of minute cytoplasmic granules.
In specimens fixed in solutions containing chromium salts, these granules stain specifically brownish yellow. This chromaffin or pheochromic reaction is thought to be due to oxidation and polymerization of the catecholamines epinephrine and norepinephrine (adrenaline and noradrenaline) within the granules.
Since these cytoplasmic organelles disrupt rapidly after death, the cytoplasm of the medullary cells of routine specimens is usually stained diffusely. The chromaffin cells can be histochemically divided into two distinct classes. Those producing epinephrine contain large amounts of acid phosphatase and stain intensely with azocarmine. However, these cells do not react with silver salts and are not autofluorescent. In contrast, the medullary cells which synthesize norepinephrine are argyrophilic and autofluorescent. They are less well stained with azocarmine, and the reaction for acid phosphatase is negative.
Kindly Let Us Know If This Was helpful? Thank You!


