What is the Dexamethasone Suppression Test (DST)?
The original protocol for assessing Cushing’s syndrome was first described in the 1960s. Liddle’s protocol involves the sequential administration of low-dose dexamethasone followed by high dose dexamethasone in patients with suspected endogenous hypercortisolemia.
Effect of Dexamethasone on the Hypothalamic-Pituitary-Adrenal (HPA) axis
Dexamethasone is the most potent exogenous glucocorticoid (up to 15x more potent than hydrocortisone) and exerts profound negative feedback inhibition of the HPA axis. More importantly, it does not bind cortisol-binding globulin, a property that allows it to traverse the blood-brain barrier easily.
Dexamethasone has no cross-reactivity with hydrocortisone and its metabolites, as such is not measurable in the standard cortisol immunoassay. This makes it an ideal exogenous glucocorticoid for dynamic testing of the HPA axis.
Procedure and interpretation of the 1mg dexamethasone suppression test
| DST | Comments |
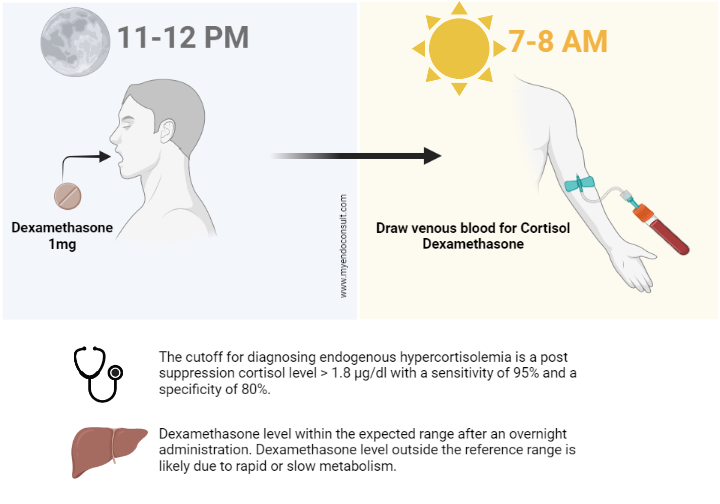
| Procedure | • Administer 1mg of dexamethasone b/n 2300-2400h • Sampling of serum cortisol b/n 0800-0900h |
| Rationale | • Dexamethasone is administered between 2300 and 2400h to inhibit the ACTH secretion, which starts at 0300h and peaks by 0700h |
| Interpretation | • The cutoff for diagnosing endogenous hypercortisolemia is > 1.8 μg/dl with a sensitivity of 95% and a specificity of 80%. • The HPA axis recovers within 24 h after the administration of a single dose of 1 mg dexamethasone. |

Drugs interfering with Dexamethasone Suppression Test
A thorough drug history is required to exclude excessive exogenous glucocorticoid exposure (leading to iatrogenic Cushing’s syndrome) before conducting biochemical testing
Causes of a false positive DST
| Category | Causes |
| Pseudo-Cushing’s syndrome | Depression, alcoholism, pregnancy, chronic kidney disease, glucocorticoid resistance syndrome, and acute stress |
| Medications associated with an increase dexamethasone metabolism | Rifampicin, phenytoin, phenobarbitone, carbamazepine, and pioglitazone |
| Medications associated with an increase in CBG | Estrogen, mitotane, and tamoxifen. |
| Unpredictable intra-individual variation | Variation in absorption and metabolism of dexamethasone can also result in false- positive DST ** |
** For this reason, assessing serum dexamethasone in addition to cortisol levels is required to detect this.
Causes of a false negative DST
| Category | Causes |
| Intermittent hypercortisolemia | Cyclical Cushing’s syndrome |
| Iatrogenic exposure to steroids | Exogenous Cushing’s syndrome |
| Inhibition of the metabolism of dexamethasone | Ritonavir, itraconazole, cimetidine, fluoxetine, and diltiazem |
When is Dexamethasone Suppression Test Required?
- Patients with unusual clinical conditions for age (e.g. early-onset osteoporosis or rapid/early-onset hypertension)
- Patients with multiple and progressive clinical features with a high pre-test probability of Cushing’s syndrome
- Patients with adrenal incidentaloma compatible with adenoma
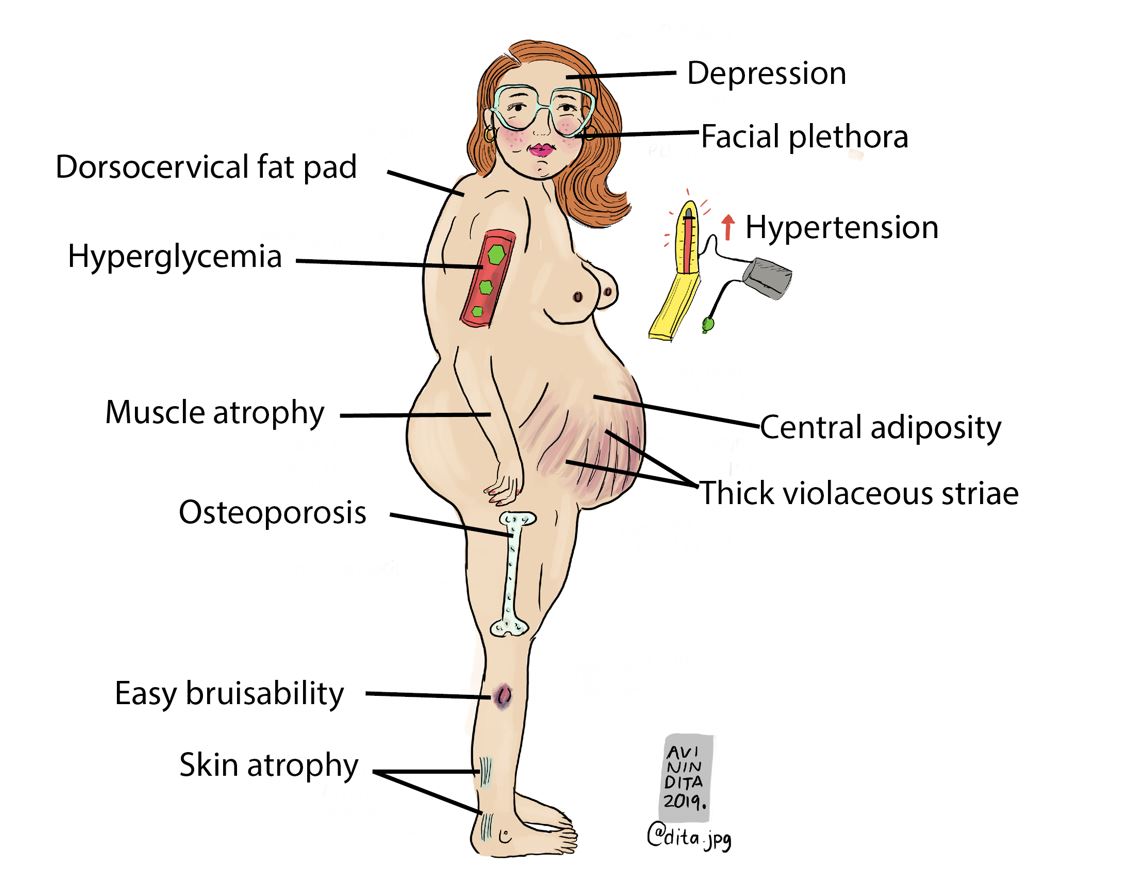
The physical examination findings that are highly suggestive of Cushing’s include proximal muscle weakness (myopathy), easy bruising, thick violaceous striae, and facial plethora.
Low Dose Dexamethasone Suppression Test (2-day test)
The standard two-day low-dose dexamethasone suppression test. Endocrinologists utilize this test to either confirm or refute equivocal screening tests such as 24-hour urinary free cortisol, late-night salivary cortisol, or the 1mg dexamethasone suppression test.
What are the optimal testing conditions for this dynamic test?
In preparing for this test, estrogens (which increase corticosteroid-binding globulin) should be discontinued. This is because estrogens raise total cortisol and result in a falsely elevated post-suppression cortisol level. Indeed, random cortisol levels in patients on estrogen are artifactually high. This can result in these patients being inadvertently referred to an endocrinologist for evaluation of suspected Cushings syndrome.
Also, medications that induce hepatic CYP3A4 may result in the rapid clearance of dexamethasone. As such, dexamethasone is unable to inhibit both ACTH and cortisol production. Post-suppression cortisol levels will therefore be inadvertently elevated (due to impaired suppression of the HPA axis), leading to a diagnosis of endogenous hypercortisolemia in normal patients without Cushing’s syndrome.
Examples of medications associated with a high false positive rate of the dexamethasone suppression test are anti-seizure drugs (e.g., phenytoin, phenobarbital, and carbamazepine) and rifampicin.
The material needed for the test is a single gold top tube. This should be labeled for the collection of serum cortisol and dexamethasone level exactly 6 hours after the last dose of dexamethasone.
So how is the two-day low-dose dexamethasone suppression test Performed?
The patient is instructed to take a 0.5mg tablet of dexamethasone every six hours over a two-day period. The classic dosing schedule involves taking dexamethasone at 8 am, 2 pm, 8 pm, and 2 am. The need to wake up during normal sleep hours makes this schedule challenging for most patients. Consequently, some endocrinologists have a slightly altered dosing schedule which involves taking the dexamethasone tablet at 6 am, 12 pm, 6 pm, and 12 am for a total of 8 doses.
Both total cortisol and post-suppression dexamethasone levels are drawn exactly 6 hours after the last dose of dexamethasone.
Interpretation of the test
It is worth noting that the 2mg 2-day dexamethasone suppression test has a higher sensitivity and specificity for diagnosing endogenous hypercortisolemia compared to the 1mg overnight dexamethasone suppression test. Post-suppression serum cortisol, which is greater than 1.4 to 1.8 mcg per dl, is highly suggestive of Cushing’s syndrome.
Summary of various forms of the Dexamethasone Suppression Test
| Type of DST | Indication | Procedure | Interpretation |
| Two-day low-dose DST | Equivocal results of 24h UFC, LNSC, or 1mg ONDST | Dexamethasone 0.5mg tablet every 6 hours x 48 hours. Early morning cortisol exactly 6 hours after the last dose of dexamethasone. | Serum cortisol >1.8 mcg/dL is suggestive of Cushing’s syndrome |
| 1mg overnight dexamethasone suppression test (low dose DST) | Evaluation of adrenal incidentaloma and screening for Cushing's syndrome | Administration of 1mg of dexamethasone at 11 pm followed by an evaluation of fasting serum cortisol at 8 am. | Serum cortisol >1.8mcg/dL is suggestive of Cushing's syndrome (screening test) |
| , or >3-5mcg/dL is suggestive of subclinical Cushing’s syndrome (adrenal incidentaloma) | |||
| 8mg overnight dexamethasone suppression test (high dose DST) | Differentiating between Cushing’s disease and ectopic ACTH production for patients with ACTH-dependent Cushing’s syndrome | Baseline serum cortisol is drawn at 8-9 am, and 8 mg of dexamethasone is administered at 11 pm. Post-suppression cortisol is then drawn around 8-9 am the next morning. | Undetectable cortisol (normal response) |
| Detectable cortisol but < 5mcg/dL (Cushing’s syndrome) | |||
| Suppression of cortisol >50% is diagnostic of Cushing’s disease. | |||
| Dexamethasone-CRH test | Differentiating between Cushing's syndrome and pseudo-Cushing’s syndrome | Dexamethasone 0.5 mg tablet every 6 hours x 48 hours. Early morning cortisol, exactly 2 hours after the last dose of dexamethasone, 100mcg of ovine CRH (oCRH) is administered as an IV bolus (8 am). Cortisol is then drawn 15 minutes after oCRH. | Post Dex-CRH cortisol of >1.4 mcg/dL suggests Cushing’s syndrome and rules out pseudo-Cushing’s syndrome. |
24h UFC 24 hour urinary free cortisol
LNSC Late-night salivary cortisol
1mg ONDST 1mg overnight dexamethasone suppression test.
‡ 8 am, 2 pm, 8 pm, and 2 am
β 12 pm, 6pm, 12am and 6am
CRH Corticotropin-releasing hormone
References
- Isidori AM, Kaltsas GA, Mohammed S, Morris DG, Jenkins P, Chew SL, Monson JP, Besser GM, Grossman AB. Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2003 Nov;88(11):5299-306. doi: 10.1210/jc.2003-030510. PMID: 14602765.
- Esfahanian F, Kazemi R. Overnight dexamethasone suppression test in the diagnosis of Cushing’s disease. Acta Med Iran. 2010 Jul-Aug;48(4):222-5. PMID: 21279933.
- Meikle AW. Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin Endocrinol (Oxf). 1982 Apr;16(4):401-8.
Kindly Let Us Know If This Was helpful? Thank You!


