The mechanism of action of various steroidogenesis inhibitors and their clinical application in managing Cushing’s disease will be reviewed.
Physiology
Adrenal steroidogenesis : Review of adrenal steroidogenesis can be found here (a simple memory device for recalling the steps in steroid synthesis). The role of various adrenal steroidogenic inhibitors such as ketoconazole, metyrapone, and mitotane is shown in figure 1.0(1).
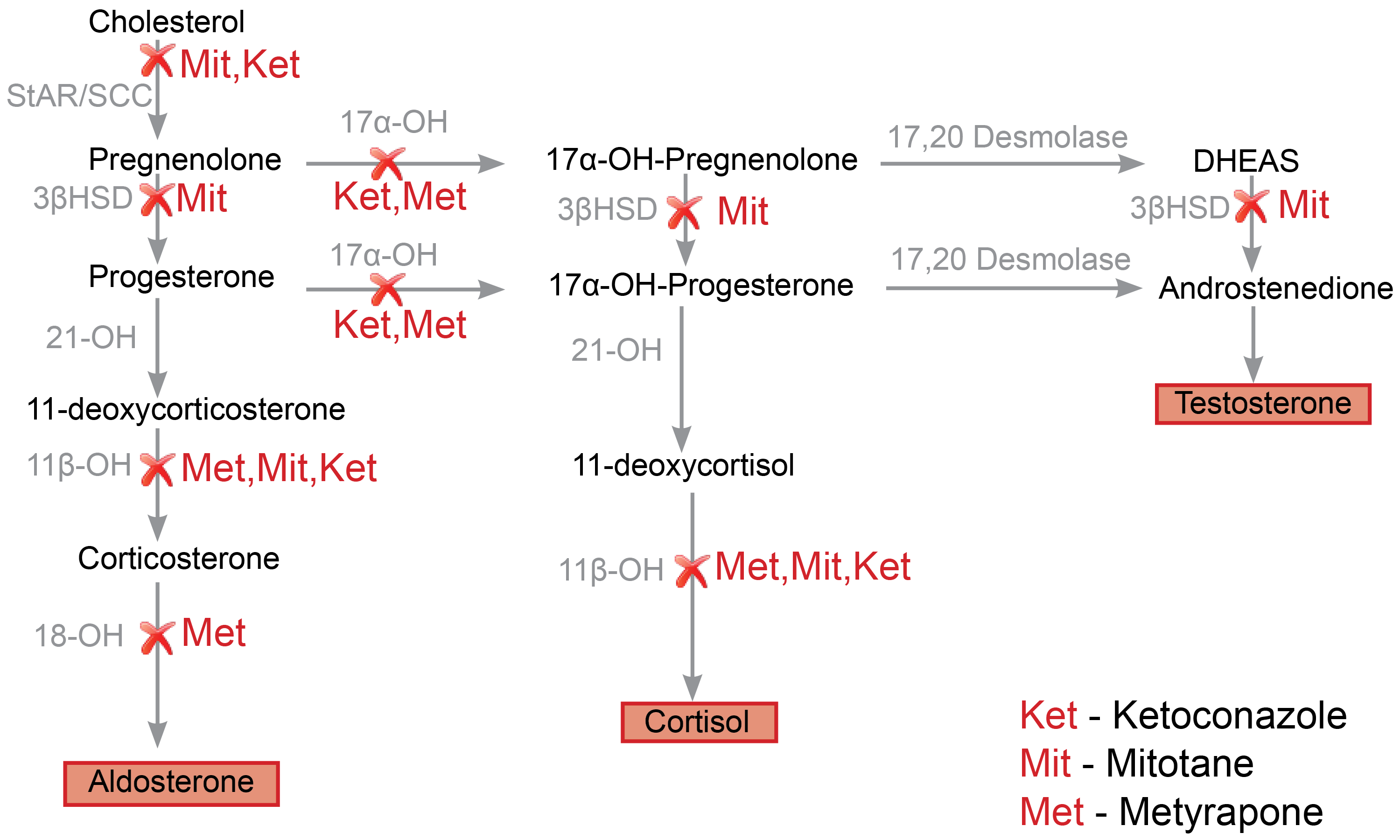
Fig. 1.0 Schematic representation of adrenal steroidogenesis pathways and the site of action of various enzyme inhibitors. Metyrapone primarily inhibits 11 beta-hydroxylase activity (11β-OH), which results in reduced production of cortisol(2). Consequently, there is an accumulation of intermediate mineralocorticoid precursors (11-DOC)(3) and shunting of progesterone and pregnenolone to androgen production(4). Mitotane inhibits various adrenal steroidogenic enzymes, including the side-chain cleavage enzyme complex(StAR/SCC), 11beta hydroxylase(11β-OH), and 3βHSD(5). Ketoconazole similarly inhibits various steroidogenic enzymes, including the side-chain cleavage enzyme complex, 17alpha hydroxylase (17α-OH), and 11β-OH enzymes(5). Redrawn and modified from Daniel E, Newell-Price JDC (2015) Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome. Eur J Endocrinol 172:R263-280
Mechanism of action
See figure 1.0 for a summary of various enzymatic targets of steroidogenic inhibitor therapies. The mechanism of action of metyrapone, mitotane, ketoconazole, and the recently approved steroidogenesis inhibitor, osilodrostat, will be reviewed next.
Metyrapone: Metyrapone has a pyridine moiety, which allows it to impair the activity of 11beta-hydroxylase (critical in the final step of cortisol synthesis). Other steroidogenic enzymes inhibited by metyrapone include the 17 alpha-hydroxylase(17α-OH) and 18-hydroxylase enzymes (less potent inhibition)(5).
Mitotane: Mitotane is suggested to have both “adrenolytic” (adrenal cell death) and “adrenostatic” (enzymatic inhibition) properties. Mitotane is a chemotherapeutic agent with a diphenylmethane moiety that causes mitochondrial dysfunction, lysis, and necrosis. Mitotane, as stated earlier, exerts its “adrenostatic” function by inhibiting side-chain cleavage enzyme, 11beta hydroxylase, and 3βHSD (5).
Ketoconazole: Ketoconazole has an imidazole group (confers its antifungal properties) with demonstrable inhibitory effects on various steroidogenesis enzymes (in particular, side-chain cleavage complex, 17 alpha-hydroxylase, and 11 beta-hydroxylase) in the adrenal cortex and gonads(5).
Osilodrostat: Osilodrostat, just like metyrapone, inhibits 11-beta hydroxylase activity in the adrenal cortex. However, unlike metyrapone, osilodrostat has a relatively higher potency and lower plasma half-life; thus, it can be administered less frequently (twice daily instead of four times daily)(6).
Practice Guide
In practice, these agents are slowly titrated to attain normal serum cortisol, which can be monitored using either late-night salivary cortisol or 24-hour urinary free cortisol. Overt hypoadrenalism is an inadvertent complication that should be anticipated in patients on steroidogenesis inhibitors(7).
Metyrapone: Hypokalemia is a known complication of treatment with metyrapone because of the accumulation of intermediate steroids with intrinsic mineralocorticoid activity (for example, 11-DOC). Close monitoring of serum potassium is therefore required(4). Furthermore, hirsutism (shunting of proximal steroids into androgenic precursors), hypertension, and edema (mineralocorticoid effects of 11-deoxycorticosterone) may occur in patients on metyrapone. Unlike ketoconazole, metyrapone is comparatively safer in pregnancy(8).
Mitotane: Mitotane is lipophilic and, as a result, is stored in an extensive repository of adipose tissue. This increases the half-life of the drug, leading to a delay in the onset of action. Most importantly, the dose of mitotane required to treat Cushing’s disease is much lower than the hefty tumoricidal dose used in the treatment of adrenocortical carcinomas(9). Mitotane has teratogenic effects, and due to its large volume of distribution (stored in adipose tissue), it should be discontinued for at least five years before potential conception(10).
Ketoconazole: Ketoconazole is the recommended first-line medical therapy for nonpregnant adults with confirmed endogenous hypercortisolemia. It is an FDA category C drug and may interfere with the androgen-dependent development of the sex organs of an unborn male baby. Despite these apparent anti-androgenic effects, it has been inadvertently used in expectant mothers without overt deleterious fetal effects(8,10). Side effects encountered in routine practice include gynecomastia, hepatic injury, male hypogonadism, and gastrointestinal discomfort. Electrolyte imbalance is usually due to either uncontrolled hypercortisolemia (mineralocorticoid receptor activation by cortisol) or hypoadrenalism(11).
Osilodrostat (isturisa): The most common adverse drug events include nausea, headaches, and the clinical effects of either the accumulation of precursor adrenal hormones or overt hypocortisolemia (adrenal insufficiency). Osilodrostat has clinical utility in persistent and recurrent Cushing’s disease(12).
Clinical Trial Evidence
Although transsphenoidal surgery (TSS) is the recommended first-line treatment for Cushing’s disease, a pituitary tumor may not be visualized in 35-65% of patients. Also, a high recurrence rate of about 20% makes medical therapies an essential adjunctive approach(9).
Key Message
Mitotane leads to biochemical amelioration of Cushing’s disease (normalization of 24-hour urinary free cortisol) in more than 70% of patients, at doses much lower than that required for the management of adrenal carcinoma.
This was a retrospective study to evaluate the efficacy of mitotane in Cushing’s disease. Seventy-six consecutive patients with proven Cushing’s disease reporting to a single facility were followed up for a median period of 6.7 months (95% CI of 5.2-8.2 months). Patients received mitotane to a total daily dose of 4 grams in 3 divided doses. Gradual de-escalation to a minimally tolerable dose needed to maintain remission was allowed during the study. There was no placebo or active comparator arm. The primary outcome was defined as remission of Cushing’s disease (normalization of 24hr urinary-free cortisol). This occurred in 72% of patients(9).
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
References
- Daniel E, Newell-Price JDC. Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome. Eur J Endocrinol. 2015 Jun;172(6):R263-280.
- Liddle GW, Estep HL, Kendall JW, Williams WC, Townes AW. Clinical application of a new test of pituitary reserve. J Clin Endocrinol Metab. 1959 Aug;19:875–94.
- Kamenický P, Droumaguet C, Salenave S, Blanchard A, Jublanc C, Gautier JF, et al. Mitotane, Metyrapone, and Ketoconazole Combination Therapy as an Alternative to Rescue Adrenalectomy for Severe ACTH-Dependent Cushing’s Syndrome. J Clin Endocrinol Metab. 2011 Sep 1;96(9):2796–804.
- Daniel E, Aylwin S, Mustafa O, Ball S, Munir A, Boelaert K, et al. Effectiveness of Metyrapone in Treating Cushing’s Syndrome: A Retrospective Multicenter Study in 195 Patients. J Clin Endocrinol Metab. 2015 Nov;100(11):4146–54.
- Pivonello R, De Leo M, Cozzolino A, Colao A. The Treatment of Cushing’s Disease. Endocr Rev. 2015 Aug 10;36(4):385–486.
- Fleseriu M, Pivonello R, Young J, Hamrahian AH, Molitch ME, Shimizu C, et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing’s disease. Pituitary. 2016 Apr;19(2):138–48.
- Tritos NA, Biller BMK. Advances in the Medical Treatment of Cushing Disease. Endocrinol Metab Clin North Am. 2020 Sep 1;49(3):401–12.
- Bronstein M, Machado M, Fragoso M. Management of pregnant patients with Cushing’s syndrome. Eur J Endocrinol. 2015;173(2):R85-91.
- Baudry C, Coste J, Khalil RB, Silvera S, Guignat L, Guibourdenche J, et al. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol. 2012 Oct 1;167(4):473–81.
- Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing’s Syndrome during Pregnancy: Personal Experience and Review of the Literature. J Clin Endocrinol Metab. 2005 May 1;90(5):3077–83.
- Luque-Ramírez M, Ortiz-Flóres AE, Nattero-Chávez L, Escobar-Morreale HF. Apparent mineralocorticoid excess as a side effect of ketoconazole therapy in a patient with Cushing’s disease. Clin Endocrinol (Oxf). 2020 Jan;92(1):80–3.
- Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020 Sep 1;8(9):748–61.
Kindly Let Us Know If This Was helpful? Thank You!


