The mechanism of action of retinoic acid and its clinical application in the management of Cushing’s disease will be reviewed.
Physiology
Regulation of corticotroph physiology by retinoic acid : The normal corticotroph cell has a POMC promotor gene, critical in POMC synthesis and eventual ACTH secretion. In normal physiology, there are retinoid-sensitive intermediary mediators (transcription factors) of POMC promotor gene activation, namely, activator protein 1 (AP-1) and nuclear receptor 77 (Nur77). Retinoic acid (RA), by binding to its nuclear retinoic acid receptors inhibits AP-1, and Nur77 expression, thus preventing POMC promotor gene activation(1,2). It is worth noting that chicken ovoalbumin upstream promoter transcription factor 1 (COUP-TF1) shields AP-1 and Nur77 from direct inactivation by RA(3). See figure 1.0. The reduced expression of the “protective transcription factor,” COUP-TF1 by some corticotroph tumoral cells, makes RA a reasonable therapeutic option in Cushing’s disease(1,3).
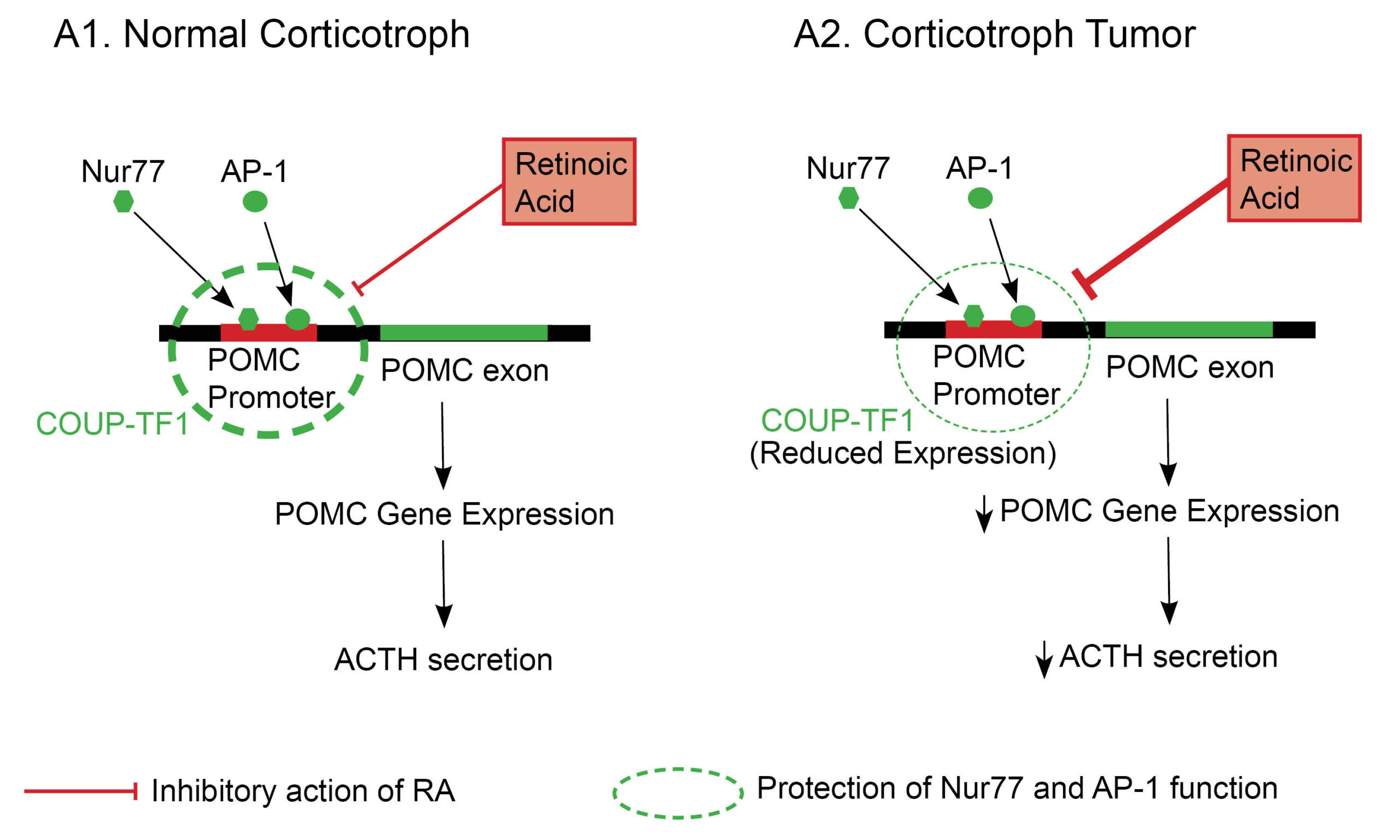
Fig. 1.0 The role of retinoic acid in anterior corticotroph pathophysiology. AP-1 and Nur77 are critical transcription factors required for POMC promotor gene activation in a corticotroph cell. The COUP-TF1 transcription factor prevents RA’s inhibitory action on both AP-1 and Nur77, allowing POMC transcription and ACTH secretion to proceed in normal physiology(image A1)(1). In contrast, corticotroph tumors have a reduced expression of COUP-TF1, which allows RA to inhibit AP-1 and Nur77 (critical mediators of POMC promotor gene activation) (image A2)(3). The thickness of the dashed circle = Degree of expression of COUP-TF1. Adapted and modified from Pecori GF et al. (2012) Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab 97:3577–3583
Mechanism of action
RA reduces cortisol synthesis in subjects with Cushing’s disease through various observed mechanistic pathways.
- RA reduces both ACTH and POMC synthesis in corticotroph tumors(3). This was reviewed earlier.
- Additionally, RA has direct tumoricidal effects on corticotroph tumors(3).
- RA reduces adrenal cortisol production through its antiproliferative effects on hyperplastic adrenocortical cells(3).
- RA also downregulates the expression of the melanocortin-2 receptor (MCR-2) by adrenal cells(4).
Practice Guide
- Retinoic acid is teratogenic and should be used with extra caution in women in the reproductive age group. Other reported side effects of RA include photosensitivity and mucositis(5).
- RA receptor activation augments the cortisol suppressive effects of Dopaminergic agonists (DAs). Combining RA and DA is a suggested therapeutic option in patients with Cushing’s disease(6).
Clinical Trial Evidence
The first proof-of-concept study of RA in humans with Cushing’s disease was carried out in 7 subjects, with a variable decrease in UFC levels, ranging from 22 to 73%(3). A recent open-label prospective trial evaluated the safety and efficacy of isotretinoin (13-cis-isomer of RA) in patients with persistent or recurrent Cushing’s disease(7).
Key Message
Retinoic acid (a novel treatment approach) was associated with a greater than 50% reduction in urinary-free cortisol levels compared to baseline in this small study. Retinoic acid is a potential therapeutic option in patients with persistent or recurrent Cushing’s disease.
In this open-label, single-arm, prospective study over a 12-month period, 16 patients with persistent or recurrent Cushing’s disease after transsphenoidal surgery were managed with isotretinoin monotherapy. All subjects received oral isotretinoin 20mg once daily. This was increased by 20mg every 4 weeks to a maximum dose of 80mg once daily.
The primary outcome was defined as either normalization of urinary free cortisol or >50% reduction in urinary free cortisol. At the end of the study, 4 patients (25%) presented with sustained normalization of UFC(7). COUP-TF1 expression (determinant of response to RA) by pituitary corticotrophs was not assessed in this study. This may explain the low response rate observed in the study
References
- Sano T. The 11th Meeting of the International Pituitary Pathology Society October 16–20, 2009 Awaji Island, Japan. Endocr Pathol. 2010 Mar 1;21(1):48–68.
- Mason D, Hassan A, Chacko S, Thompson P. Acute and chronic regulation of pituitary receptors for vasopressin and corticotropin releasing hormone. Arch Physiol Biochem. 2002 Apr;110(1–2):74–89.
- Pecori Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Karamouzis I, et al. Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab. 2012 Oct;97(10):3577–83.
- Sesta A, Cassarino MF, Tapella L, Castelli L, Cavagnini F, Pecori Giraldi F. Effect of retinoic acid on human adrenal corticosteroid synthesis. Life Sci. 2016 Apr 15;151:277–80.
- Theodoropoulou M, Reincke M. Tumor-Directed Therapeutic Targets in Cushing Disease. J Clin Endocrinol Metab. 2019 Mar 1;104(3):925–33.
- Occhi G, Regazzo D, Albiger NM, Ceccato F, Ferasin S, Scanarini M, et al. Activation of the dopamine receptor type-2 (DRD2) promoter by 9-cis retinoic acid in a cellular model of Cushing’s disease mediates the inhibition of cell proliferation and ACTH secretion without a complete corticotroph-to-melanotroph transdifferentiation. Endocrinology. 2014 Sep;155(9):3538–49.
- Vilar L, Albuquerque JL, Lyra R, Trovão Diniz E, Rangel Filho F, Gadelha P, et al. The Role of Isotretinoin Therapy for Cushing’s Disease: Results of a Prospective Study. Int J Endocrinol. 2016;2016:8173182.
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
Kindly Let Us Know If This Was helpful? Thank You!


