Introduction
Immune checkpoint inhibitors enhance the immune system’s ability to recognize and attack cancer cells. They target specific proteins on immune cells, called immune checkpoints, which play a critical role in modulating the immune response[1].
Under normal conditions, immune checkpoints help maintain self-tolerance and prevent the immune system from causing damage to healthy cells[2]. However, cancer cells can exploit these checkpoints to evade immune surveillance and destruction by expressing inhibitory proteins that bind to checkpoint receptors on immune cells, such as T cells. This binding effectively “switches off” the immune response against the cancer cells[3].
Immune checkpoint inhibitors work by blocking the interaction between checkpoint proteins and their ligands, thereby releasing the “brakes” on the immune system and allowing T cells to recognize and attack cancer cells more effectively. Some examples of targeted immune checkpoints include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1)[4,5].
Immune checkpoint inhibitors have shown promising results in treating various cancers, including melanoma, non-small cell lung cancer, and renal cell carcinoma, among others[6]. However, they can also cause immune-related adverse events due to increased immune activation, potentially leading to inflammation and damage to healthy tissues. The endocrine system is susceptible to these immune-related adverse events[7].
Immune regulation and cancer
CTLA-4 is expressed on the surface of T cells and competes with the co-stimulatory receptor CD28 for binding to both CD80 and CD86, present on antigen-presenting cells (APCs)[8]. While CD28 binding enhances T cell activation, CTLA-4 binding inhibits T cell activation. In cancer, overexpression of CTLA-4 can dampen the immune response against tumor cells by inhibiting T-cell activation, thus promoting tumor progression[9].
PD-1 is also expressed on the surface of T cells and binds to its ligands[10], programmed death ligand 1 (PD-L1), and programmed death ligand 2 (PD-L2), which can be found on tumor cells and some immune cells[11]. The binding of PD-1 to its ligands leads to the suppression of T cell activation and proliferation, promoting immune tolerance and allowing tumor cells to effectively evade immune detection[12].
Immune checkpoint inhibitors represent a new class of anti-cancer medications that have been developed to target these immune checkpoints and counteract the immunosuppressive effects of CTLA-4 and PD-1 activation pathways.
Anti-CTLA-4 inhibitors, such as ipilimumab, disrupt the binding of CTLA-4 to CD80/CD86, preventing the inhibition of T cell activation and promoting an anti-tumor immune response[13].
Also, anti-PD-1 inhibitors, like nivolumab and pembrolizumab, block the interaction between PD-1 and its ligands, allowing T cells to maintain their activation and attack tumor cells[14]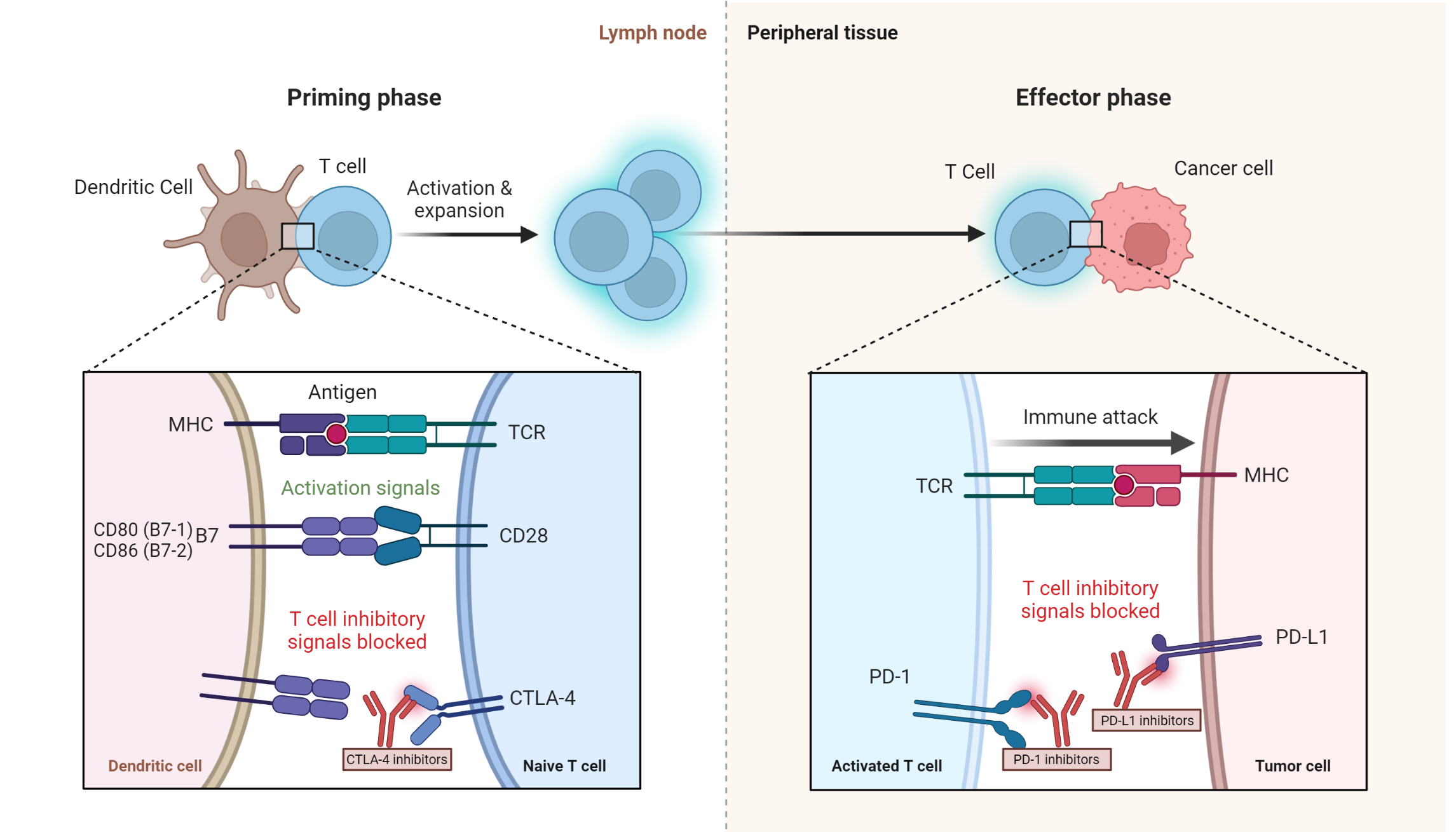
Figure 1. Mechanism of action of various immune check inhibitors. The phases of immune checkpoint processes, showing the role of the dendritic cell, T cell and cancer cell during the priming and effector phases. The site of action of immune checkpoint inhibitors (CTLA-4 inhibitors, PD-1 inhibitors and PD-L1 inhibitors) is shown.
Table 1. Sites of action of immune checkpoint inhibitors
| Immune checkpoint inhibitors | Site of action |
| Atezolizumab, Avelumab, Durvalumab[15] | PD-L1 |
| Nivolumab, Pembrolizumab, Cemiplimab, Dostarlimab | PD-1 |
| Ipilimumab, Tremelimumab[16] | CTLA-4 |
Pathophysiology pearl
The proposed mechanisms of toxicity (irAEs) associated with ICIs
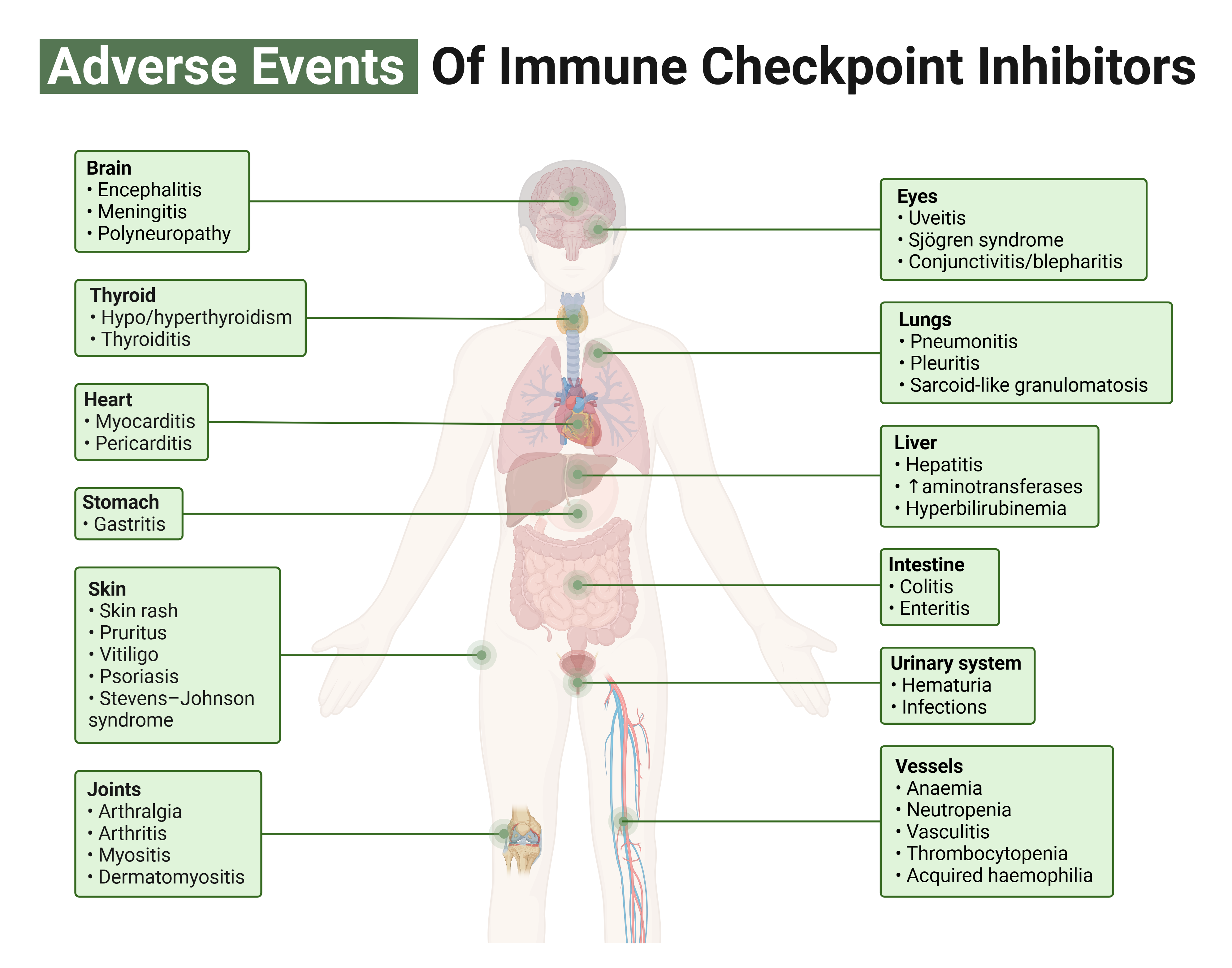
Immune-related adverse events in the setting of ICIs can affect any organ or tissue, with the endocrine system being no exception.
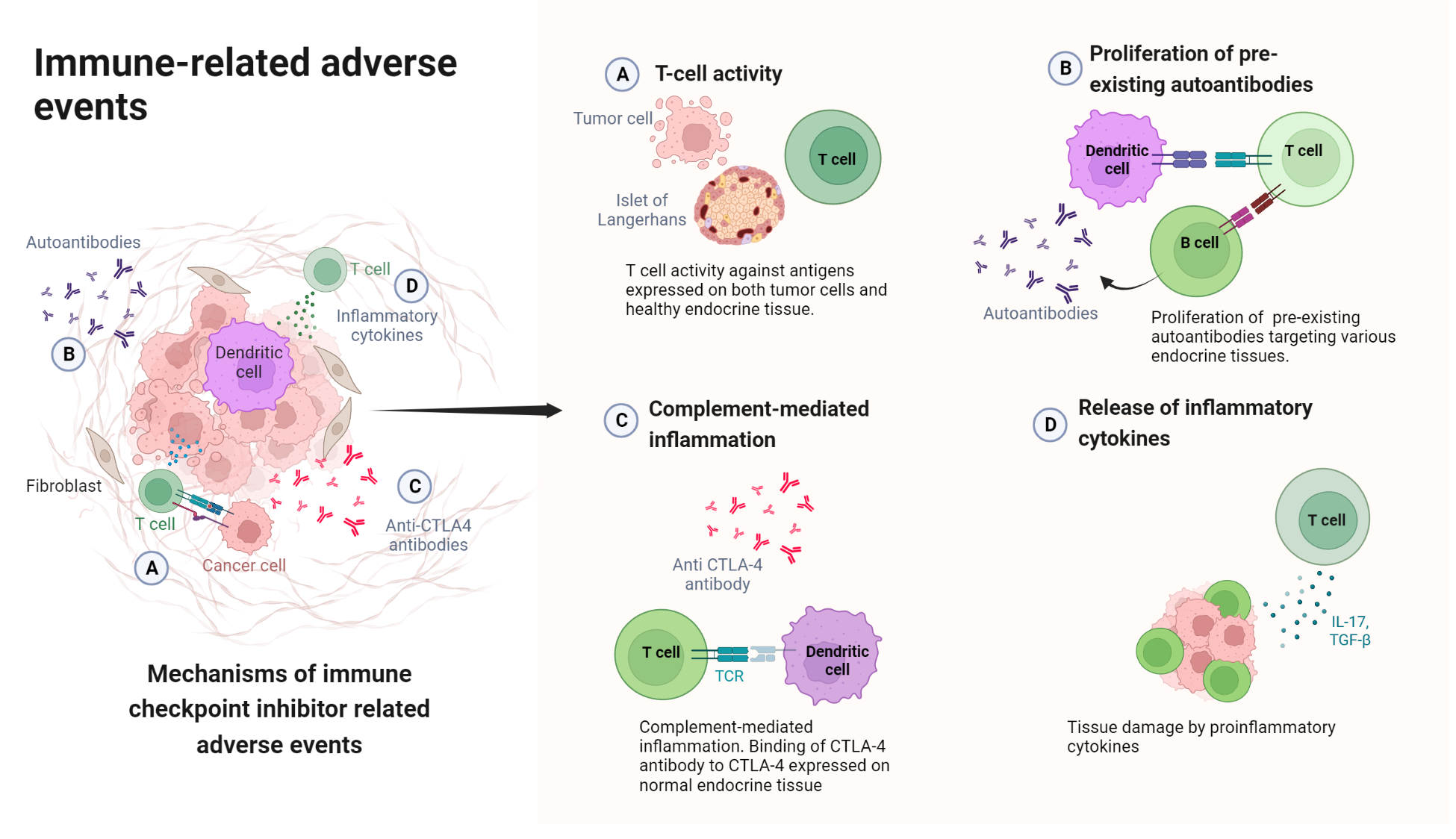
Figure 2. Mechanisms of immune checkpoint inhibitor-related adverse events. The proposed mechanisms include T cell activity against antigens on normal endocrine cells, proliferation of pre-existing antibodies, complement activation, and the release of proinflammatory cytokines[17].
Endocrine effects of immune checkpoint inhibitors
Hypophysitis – An immune-related adverse reaction
Immune checkpoint inhibitors have been associated with various immune-related adverse events (irAEs), one of which is hypophysitis. Hypophysitis can result in the disruption of normal hormone production and regulation[18].
The association between immune checkpoint inhibitors and hypophysitis is thought to be due to the enhanced immune activation caused by these drugs. By blocking inhibitory immune checkpoints, such as CTLA-4 and PD-1/PD-L1, immune checkpoint inhibitors enable a more potent immune response against cancer cells. However, this heightened immune activation can also lead to the immune system mistakenly targeting and attacking healthy tissues, such as the pituitary gland, causing inflammation and subsequent dysfunction[19,20].
Hypophysitis is more commonly observed with CTLA-4 inhibitors, such as ipilimumab, compared to PD-1 and PD-L1 inhibitors, like nivolumab and pembrolizumab. Nonetheless, the risk of hypophysitis should be considered when using any immune checkpoint inhibitors, and patients should be closely monitored for signs and symptoms[21,22]. Hypophysitis is more common with CTLA-4 inhibitors than PD-1/PD-L1 inhibtors[23].
Thyroid Dysfunction
Immune checkpoint inhibitors can cause thyroid dysfunction, which can manifest as hypothyroidism or overt hyperthyroidism[24]. This immune response can result in the destruction of thyroid tissue and the development of hypothyroidism or, initially, transient hyperthyroidism due to the release of preformed thyroid hormones through a process of destructive thyroiditis[25,26].
Also, some tumor antigens might share structural similarities with thyroid antigens, leading to the activation of T cells that cross-react with both tumor and thyroid tissue (molecular mimicry). This cross-reactivity could result in an autoimmune attack on the thyroid gland, causing thyroid dysfunction[26,27].
Autoimmune Diabetes
Immune checkpoint inhibitors (ICIs) have been reported to cause autoimmune diabetes, also known as insulin-dependent diabetes or type 1 diabetes mellitus, as a rare immune-related adverse event (irAE)[28]. This occurs when the immune system mistakenly attacks and destroys insulin-producing beta cells in the pancreas, leading to insulin deficiency and hyperglycemia. The precise mechanism by which ICIs induce autoimmune diabetes is not yet fully understood[29].
Hypoparathyroidism
The development of hypoparathyroidism due to immune checkpoint inhibitors (ICIs) is a rare immune-related adverse event (irAE).
Early detection and management of hypoparathyroidism in patients receiving ICIs are essential to prevent complications, such as hypocalcemia and associated symptoms like muscle cramps, tetany, and seizures. Patients should be monitored for symptoms of hypoparathyroidism and serum calcium levels, and appropriate treatment, such as calcium and vitamin D supplementation, should be initiated if necessary.
References
1. Darvin, P., Toor, S.M., Sasidharan Nair, V., and Elkord, E. (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med, 50 (12), 1–11.
2. Lee, L., Gupta, M., and Sahasranaman, S. (2016) Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J Clin Pharmacol, 56 (2), 157–169.
3. Hargadon, K.M., Johnson, C.E., and Williams, C.J. (2018) Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol, 62, 29–39.
4. Shiravand, Y., Khodadadi, F., Kashani, S.M.A., Hosseini-Fard, S.R., Hosseini, S., Sadeghirad, H., Ladwa, R., O’Byrne, K., and Kulasinghe, A. (2022) Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol, 29 (5), 3044–3060.
5. Marin-Acevedo, J.A., Kimbrough, E.O., and Lou, Y. (2021) Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol, 14 (1), 45.
6. Atkins, M.B., Clark, J.I., and Quinn, D.I. (2017) Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol, 28 (7), 1484–1494.
7. Cappelli, L.C., and Bingham, C.O. (2021) Spectrum and impact of checkpoint inhibitor-induced irAEs. Nat Rev Rheumatol, 17 (2), 69–70.
8. Sansom, D.M. (2000) CD28, CTLA-4 and their ligands: who does what and to whom? Immunology, 101 (2), 169–177.
9. Esensten, J.H., Helou, Y.A., Chopra, G., Weiss, A., and Bluestone, J.A. (2016) CD28 costimulation: from mechanism to therapy. Immunity, 44 (5), 973–988.
10. Simon, S., and Labarriere, N. (2017) PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology, 7 (1), e1364828.
11. Philips, E.A., Garcia-España, A., Tocheva, A.S., Ahearn, I.M., Adam, K.R., Pan, R., Mor, A., and Kong, X.-P. (2020) The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. J Biol Chem, 295 (14), 4372–4380.
12. Dong, Y., Sun, Q., and Zhang, X. (2016) PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget, 8 (2), 2171–2186.
13. Vandenborre, K., Van Gool, S.W., Kasran, A., Ceuppens, J.L., Boogaerts, M.A., and Vandenberghe, P. (1999) Interaction of CTLA‐4 (CD152) with CD80 or CD86 inhibits human T‐cell activation. Immunology, 98 (3), 413–421.
14. Fessas, P., Lee, H., Ikemizu, S., and Janowitz, T. (2017) A molecular and preclinical comparison of the PD-1–targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol, 44 (2), 136–140.
15. Rotte, A. (2019) Combination of CTLA-4 and PD-1 blockers for treatment of cancer. Journal of Experimental & Clinical Cancer Research, 38 (1), 255.
16. Seidel, J.A., Otsuka, A., and Kabashima, K. (2018) Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Frontiers in Oncology, 8.
17. Postow, M.A., Sidlow, R., and Hellmann, M.D. (2018) Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med, 378 (2), 158–168.
18. Mahzari, M., Liu, D., Arnaout, A., and Lochnan, H. (2015) Immune Checkpoint Inhibitor Therapy Associated Hypophysitis. Clin Med Insights Endocrinol Diabetes, 8, 21–28.
19. Mortensen, M.J., Oatman, O., Azadi, A., Fonkem, E., and Yuen, K.C.J. (2020) An Update on Immune Checkpoint Inhibitor-related Hypophysitis.
20. Albarel, F., Gaudy, C., Castinetti, F., Carré, T., Morange, I., Conte-Devolx, B., Grob, J.-J., and Brue, T. (2015) Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. European Journal of Endocrinology, 172 (2), 195–204.
21. Nguyen, H., Shah, K., Waguespack, S.G., Hu, M.I., Habra, M.A., Cabanillas, M.E., Busaidy, N.L., Bassett, R., Zhou, S., Iyer, P.C., Simmons, G., Kaya, D., Pitteloud, M., Subudhi, S.K., Diab, A., and Dadu, R. (2021) Immune checkpoint inhibitor related hypophysitis: diagnostic criteria and recovery patterns. Endocrine-Related Cancer, 28 (7), 419–431.
22. Kotwal, A., Rouleau, S.G., Dasari, S., Kottschade, L., Ryder, M., Kudva, Y.C., Markovic, S., and Erickson, D. (2022) Immune checkpoint inhibitor-induced hypophysitis: lessons learnt from a large cancer cohort. Journal of Investigative Medicine, 70 (4), 939–946.
23. Di Dalmazi, G., Ippolito, S., Lupi, I., and Caturegli, P. (2019) Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab, 14 (6), 381–398.
24. Iwama, S., Kobayashi, T., Yasuda, Y., and Arima, H. (2022) Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab, 36 (3), 101660.
25. Muir, C.A., Wood, C.C.G., Clifton-Bligh, R.J., Long, G.V., Scolyer, R.A., Carlino, M.S., Menzies, A.M., and Tsang, V.H.M. (2022) Association of Antithyroid Antibodies in Checkpoint Inhibitor-Associated Thyroid Immune-Related Adverse Events. J Clin Endocrinol Metab, 107 (5), e1843–e1849.
26. Chera, A., Stancu, A.L., and Bucur, O. (2022) Thyroid-related adverse events induced by immune checkpoint inhibitors. Frontiers in Endocrinology, 13.
27. Zhan, L., Feng, H., Liu, H., Guo, L., Chen, C., Yao, X., and Sun, S. (2021) Immune Checkpoint Inhibitors-Related Thyroid Dysfunction: Epidemiology, Clinical Presentation, Possible Pathogenesis, and Management. Frontiers in Endocrinology, 12.
28. Kyriacou, A., Melson, E., Chen, W., and Kempegowda, P. (2020) Is immune checkpoint inhibitor-associated diabetes the same as fulminant type 1 diabetes mellitus? Clin Med (Lond), 20 (4), 417–423.
29. Chen, X., Affinati, A.H., Lee, Y., Turcu, A.F., Henry, N.L., Schiopu, E., Qin, A., Othus, M., Clauw, D., Ramnath, N., and Zhao, L. (2022) Immune Checkpoint Inhibitors and Risk of Type 1 Diabetes. Diabetes Care, 45 (5), 1170–1176.
Kindly Let Us Know If This Was helpful? Thank You!


