Introduction
Primary amenorrhea is a multifaceted clinical problem that affects approximately 1-3% of adolescent girls. It can be the result of various congenital or acquired conditions, including genetic disorders, anatomic abnormalities, endocrine dysfunctions, and systemic diseases. Prompt and accurate evaluation is vital in identifying the underlying cause and initiating the appropriate treatment, ultimately improving the patient’s reproductive health and overall quality of life.
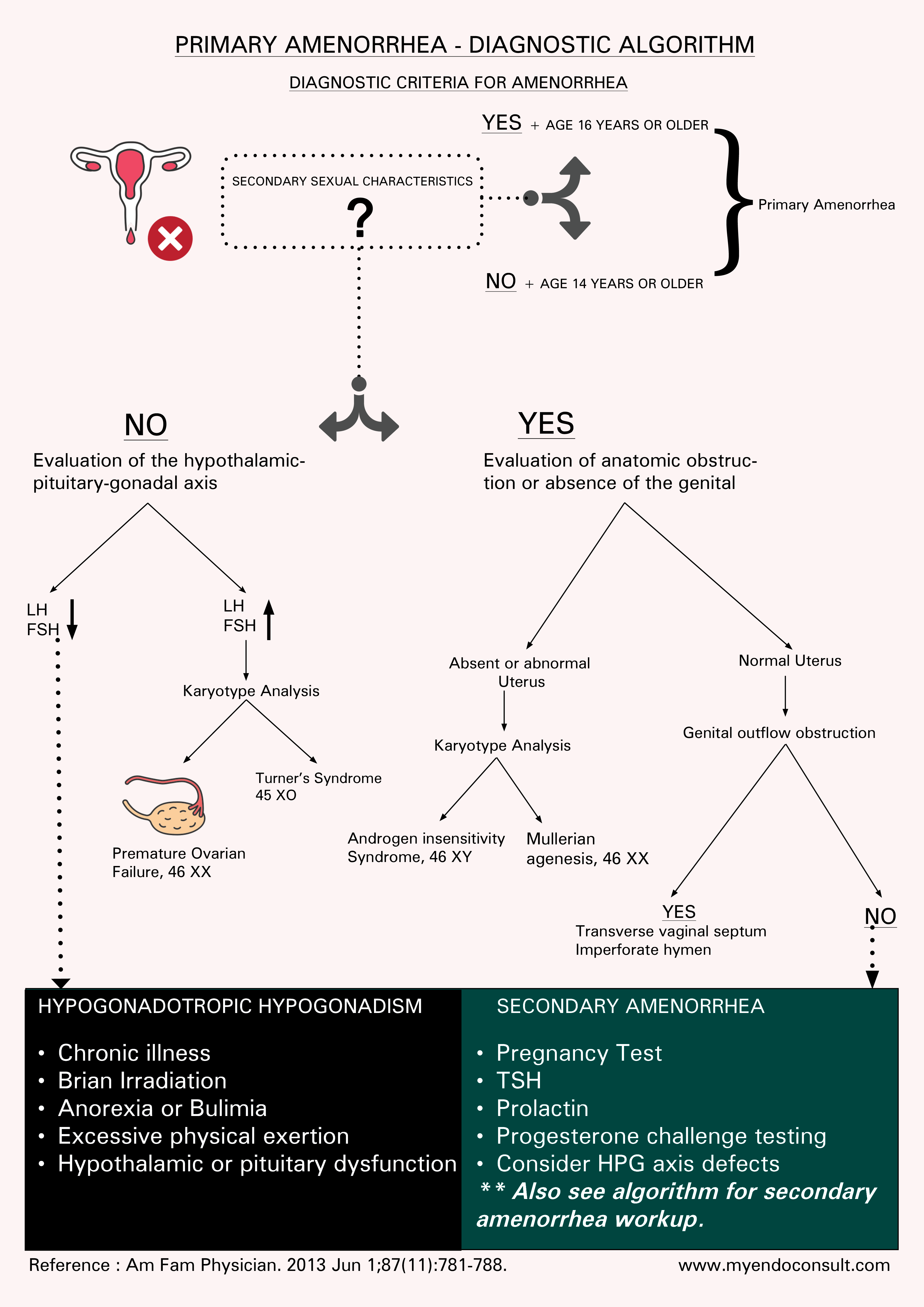
Primary Amenorrhea – Algorithm
Diagnosis of primary amenorrhea in the following clinical scenarios. A urine pregnancy test, TSH and serum prolactin level should be completed prior to further evaluation, due to the possibility of pregnancy, hypothyroidism or a prolactinoma being the underlying cause of amenorrhea.
- Amenorrhea + Lack of secondary sexual characteristics in a female aged 14 years or older.
- Amenorrhea + Presence of secondary sexual characteristics in a female 16 years or older.
Physiology Of Menstrual Regulation
The proliferative and secretory phases of the endometrial cycle are controlled by ovarian hormones, principally 17β-estradiol, and progesterone. These steroid hormones originate from the developing antral follicles and the corpus luteum during the follicular and luteal phases.
The hypothalamic-hypophyseal axis essentially manages the generation of these hormones. This system includes hypothalamic neurons that produce the polypeptide gonadotrophin-releasing hormone (GnRH) and two glycoprotein hormones, FSH and LH, which are created by the anterior pituitary's gonadotrophs.
Crucially, gonadal steroids exert feedback effects at both hypothalamic and adenohypophyseal levels and other influences that affect gonadal steroid production.
The release of GnRH, like other hypothalamic-releasing hormone neurosecretions, is periodic and essential to effectively instigating the release of LH and FSH. Continuous infusion of GnRH initially stimulates, but then suppresses, the release of these gonadotropins.
Each menstrual cycle starts (from day 1 to 5) due to reduced gonadal steroid production. This decrease leads spiral blood vessels to vasoconstrict, severely reducing blood flow to the endometrium and causing necrosis of the outer endometrial layers, leading to their shedding and menstruation.
Coupled with the reduction in gonadal steroid production is a decrease in negative feedback on the hypothalamo-adenohypophysial axis. As a result, there is an increase in the release of GnRH pulses from hypothalamic neurons and a subsequent increase in LH and FSH release from the anterior pituitary.
These adenohypophysis hormones, especially FSH, stimulate the development of about 20 preantral follicles in the ovaries. These follicles begin the synthesis of 17β-estradiol, as the well as precursor androgens and inhibin. The concentration of these substances does not escalate just in the expanding follicles and the general circulation, increasing the negative feedback on the production of LH and FSH.
As FSH levels decrease, the antral follicles that were growing under the influence of FSH are no longer adequately stimulated to continue developing, so their growth ceases and they enter the atrophy process known as atresia. The typically surviving solitary follicle (the Graafian follicle) continues to grow due to the stimulating effect of the oestradiol, which is now being produced in large quantities.
Granulosa cells have generated estrogen receptors, enabling the follicle to continue growing and developing through a paracrine effect, producing even more oestradiol. As indicated in the section discussing the ovarian cycle, if the circulating estradiol concentration is sufficiently high for a long enough period (around 36 hours), it suddenly imparts a positive feedback effect on the hypothalamic pulsatile release of GnRH and also enhances the sensitivity of the gonadotrophs to GnRH.
As a result, the generation of LH and, to a smaller extent FSH, is stimulated, causing a surge in their concentrations within the circulation. LH surge, in particular, is associated with ovum final maturation and the final stages of follicular development, culminating in the release of the ovum during ovulation. This event ends the ovarian follicular phase, which sees proliferation of the endometrium under the influence of estradiol, which is the proliferative phase of the endometrial cycle.
The level of estradiol now decreases and the cells of the collapsed follicle are transformed into luteal cells. The corpus luteum begins to produce estradiol and progesterone under the influence of increasing levels of LH and FSH. This phase, known as the luteal phase of the ovarian cycle, aligns with the stimulation of endometrial secretions by increasing levels of both oestradiol and progesterone, which is the secretory phase of the endometrial cycle.
If the ovum remains unfertilized, the increasing levels of gonadal steroids exert an increasing negative feedback on the hypothalamo-hypophysial axis. This leads to a reduction in the pulsatile release of GnRH and, consequently, a decrease in the levels of circulating gonadotrophins. As LH and FSH levels drop, stimulation of the luteal cells decreases, causing a subsequent decrease in estradiol and progesterone levels. The endometrium, no longer maintained by gonadal steroids, sheds its outer layers, signaling the start of the next cycle.
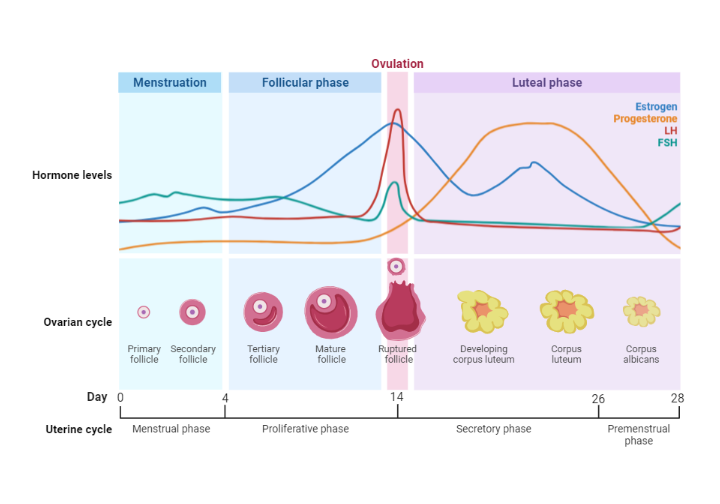
Interestingly, even if estradiol reaches the same high concentration as at the end of the follicular phase, it does not trigger another surge in LH (and FSH) due to the overriding inhibitory influence of the high circulating concentration of progesterone, which was absent earlier. The influence of other hormones also merits consideration. Inhibin, in particular, is likely to play a significant negative feedback role during the menstrual cycle. Synthesized by granulosa cells during the ovarian cycle, inhibin specifically inhibits FSH through a negative feedback mechanism. It is plausible that the decrease in FSH production during the midfollicular phase is at least partly due to the effect on gonadotrophs by inhibiting the action of locally produced activin. This results in an inhibition of FSH synthesis.
Suppose that the ovum released during ovulation is fertilized by a sperm cell. In that case, a continuous need for gonadal steroids arises as endometrial secretory activity must be sustained, and other tissues, such as mammary tissue, require these hormones for their development. Given that increasing ovarian gonadal steroids inhibit the maternal hypothalamo-adenohypophysial axis, a new stimulus for their production is necessary. This stimulus initially comes from the implanting blastocyst, which begins to produce a hormone, human chorionic gonadotrophin (hCG), that can bind to LH receptors on corpus luteum cells.
Multiple other factors also affect the production of gonadal steroids. Body weight is an evident factor; A decrease in Body Mass Index (BMI) below the normal range (i.e. less than 19) may lead to oligomenorrhoea or amenorrhea (disturbed or absent menstrual cycles, respectively). Therefore, medical conditions such as anorexia nervosa or significant weight loss linked to prolonged extreme exercise can disrupt menstrual cycles. Intriguingly, the onset of puberty, especially in girls, is also associated with achieving a certain critical body weight. Hypothalamic polypeptide kisspeptin has been associated with the beginning of adult-like pulses of GnRH neurons in puberty.
Disrupted cycles can also be associated with other endocrine disorders, such as hypothyroidism and hypercortisolism (Cushing syndrome), suggesting that iodothyronines stimulate and glucocorticoids inhibit the hypothalamic-adenohypophysial-ovarian axis. Due to its inhibitory effect on GnRH release, hyperprolactinemia is also associated with loss of menstrual cycles. Thus, it can also be inhibitory. Endorphins can also suppress the production of gonadal hormones. Many of these endocrine factors probably impact the pulsatile release of GnRH, but may also affect pituitary gonadotrophs.
Evaluation of primary amenorrhea
Evaluation of primary amenorrhea involves a systematic approach that begins with a comprehensive medical history, followed by a detailed physical examination, appropriate laboratory tests, and imaging studies guided by the initial findings.
Medical history should include questions about the patient's age, growth and development, exercise habits, nutritional status, and signs of systemic disease. A family history of delayed puberty, infertility, or other gynecological problems is also significant. A history of anosmia may indicate Kallmann syndrome, while galactorrhea may suggest hyperprolactinemia.
A detailed physical examination, including assessing the patient's height, weight, and body mass index (BMI), is essential. Evaluating the development and progression of secondary sexual characteristics using the Tanner staging system can provide valuable information on the patient's pubertal status. Careful examination of features suggestive of specific syndromes, such as Turner syndrome (short stature, webbed neck, broad chest with widely spaced nipples), is also vital.
Basic laboratory tests should include a pregnancy test to rule out the possibility of cryptic pregnancy. Additional tests should be tailored based on initial findings, but they commonly include levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol, prolactin, and thyroid stimulating hormone (TSH) levels. Elevated FSH levels may indicate ovarian failure, while low levels of FSH and LH may suggest a hypothalamic or pituitary disorder. A karyotype analysis should be considered in patients with high levels of FSH or physical findings suggestive of a chromosomal disorder.
Imaging studies, such as ultrasound or magnetic resonance imaging (MRI), can help visualize reproductive anatomy and detect anatomical abnormalities, such as Müllerian agenesis or androgen insensitivity syndrome. An MRI may also be necessary to evaluate hypothalamic or pituitary lesions in cases of suspected central causes of amenorrhea.
Clinical implications and Management
Diagnostic evaluation of primary amenorrhea is crucial for determining the cause and guiding appropriate treatment. It allows early detection of serious conditions such as Turner syndrome or Müllerian agenesis, which require a specific therapeutic approach. Treatment may include hormone replacement therapy, surgery for anatomical abnormalities, or lifestyle modifications for weight-related or exercise.
Furthermore, early diagnosis can help alleviate psychological distress often associated with primary amenorrhea and address potential long-term complications such as osteoporosis, infertility, or cardiovascular disease in specific conditions.
Conclusion
Primary amenorrhea is a complex condition that requires a comprehensive and systematic evaluation to identify its underlying cause. Through a detailed medical history, detailed physical examination, appropriate laboratory tests, and imaging studies, healthcare providers can accurately diagnose the condition, initiate an appropriate therapeutic plan, and ultimately improve the patient's reproductive health and quality of life. Effective treatment of primary amenorrhea requires an integrated, patient-centered approach that also considers the psychosocial implications of the condition.
References
Klein DA, Poth MA. Amenorrhea: an approach to diagnosis and management. Am Fam Physician. 2013 Jun 1;87(11):781-8.
Kindly Let Us Know If This Was helpful? Thank You!


