The mechanism of action of bromocriptine and cabergoline and their clinical application in the management of prolactinomas will be reviewed.
Physiology
Regulation of prolactin release : Anterior pituitary lactotrophs release prolactin (a peptide hormone) under trophic stimulation by a hypothalamic-derived prolactin-releasing factor (a putative hormone), vasoactive intestinal peptide, or thyrotropin-releasing hormone(TRH)(1).
Dopamine from the hypothalamus (tuberoinfundibular dopaminergic neuronal cells) inhibits prolactin release by binding to D2 receptors on anterior pituitary lactotrophs. Prolactin increases hypothalamic dopamine release by upregulating tyrosine hydroxylase activity (dopamine synthesis pathway) in tuberoinfundibular neurons, thus, promoting its inhibition by dopamine(2).
Table 1.0 Regulators of prolactin secretion
| Inhibition of prolactin secretion | Stimulation of prolactin secretion |
| Dopamine | TRH |
| Somatostatin | VIP |
| GABA | Oxytocin |
| Glucocorticoids | Vasopressin |
VIP Vasoactive intestinal peptide, GABA Gamma-aminobutyric acid
Adapted from Saleem M et al. (2018) Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin Biochem Rev 39:3–16
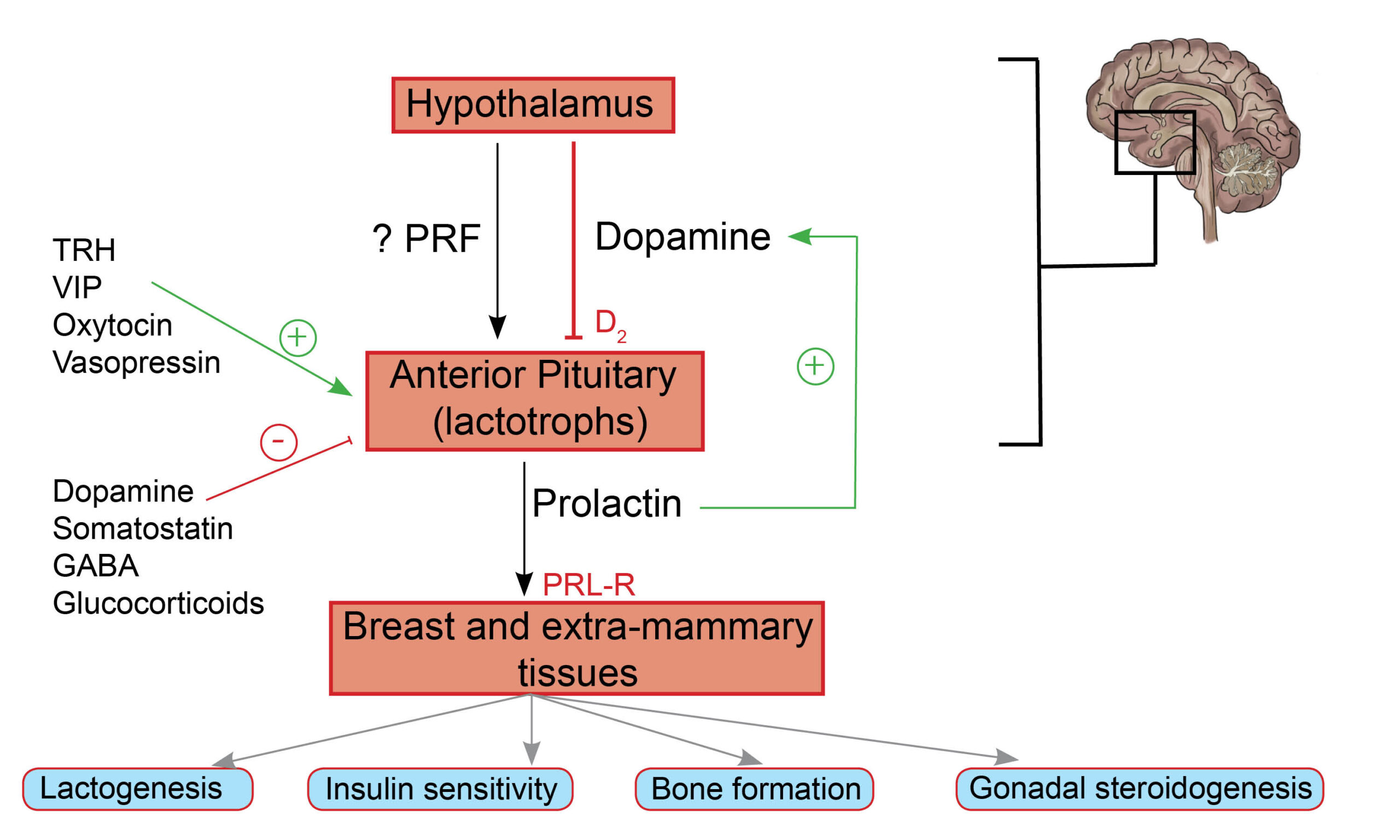
Fig. 1.0 Schematic representation of the hypothalamic-pituitary-mammary axis. PRF is a putative (unconfirmed) hormone involved in the stimulation of PRL release by lactotrophs in the anterior pituitary gland. Dopamine from the hypothalamus impairs the release of prolactin by binding and activating D2 receptors present on lactotrophs. Stimulatory and inhibitory factors involved in the regulation of PRL release are represented by + and – signs. Extramammary tissues include gonadal steroidogenesis, skeletal growth, and glucose metabolism, to mention a few.
Although classically associated with the function of mammary glands, prolactin has several extra-mammary effects due to the presence of prolactin receptors in various tissues. There are PRL receptors (PRL-Rs) on pancreatic beta cells (glucose-mediated insulin release), adipose tissue (thermoregulation), and hematopoietic cells (T-cell activation), to mention a few(3,4).
Mechanism of action
Dopaminergic agonists (cabergoline and bromocriptine), synthetic ergot derivatives, bind to D2 receptors on the surface of pituitary lactotroph tumors and induce dopamine-mediated inhibition of prolactin synthesis and secretion. Dopaminergic agonists also exhibit “tumoricidal properties” by promoting programmed cell death of lactotroph tumors through complex intracellular pathways involving estrogen and neuronal dopamine transporters(5).
Practice Guide
- Patients who do not experience a normalization of serum PRL or a 50% reduction in tumor size are classified as being dopaminergic agonist resistant. There is, however, no conventionally accepted dose or duration of exposure to DA required to diagnose a patient as being DA resistant (6,7).
- Predictors of inadequate response to dopaminergic agonists include male gender, macroprolactinoma, tumor characteristics (cystic or hemorrhagic), prolonged latency to euprolactinemia, and high PRL at baseline(8).
- For women in the reproductive age group who are planning a pregnancy, the use of bromocriptine is a safer therapeutic option compared to cabergoline. This is due to more safety data being available for the former compared to the latter(9).
- Side effects of DAs include nausea, vomiting, headaches, postural hypotension, psychotropic side effects (hallucinations, psychosis), and nasal congestion(10,11). The dictum, “start low and go slow,” helps mitigate the side effects of DAs. Cabergoline has a longer half-life, a higher affinity for D2Rs, and a relatively tolerable side effect profile compared to bromocriptine(12).
- It is reasonable to screen for psychiatric disorders before initiating dopaminergic agonists. Compulsive gambling has been associated with cabergoline use; it is, therefore, reasonable to alert patients of this potential side effect(13).
Clinical Trial Evidence
There was a paradigm shift from surgery to dopaminergic agonists for the management of prolactinomas in the 1970s due to the superior efficacy and safety of medical therapy compared to surgery. Dopaminergic agonists are now widely recommended as a first-line treatment option for patients with prolactinomas. Due to advancements in transsphenoidal endoscopic procedures, surgery may be a viable option for patients. This was explored in a systematic review investigating surgery as a viable alternative first-line treatment for prolactinoma(14).
Key Message
Dopaminergic agonists remain the first-line therapeutic option in most patients with prolactinomas. Surgery is, however, more likely to lead to long-term remission in patients irrespective of tumor size (macroprolactinoma or macroprolactinoma), based on the results of this large meta-analysis.
In this meta-analysis, evaluating long-term remission rates after either a dopaminergic holiday or transsphenoidal surgery, patients were grouped into medical (n=3564) and surgical (n=1836) arms. The primary outcome was defined as long-term (≥1 year) remission (in other words, maintenance of normal serum prolactin) after either the withdrawal of medical therapy or post-transsphenoidal surgery. The primary outcome after the dopaminergic holiday was 34% (95% CI, 26-46) and 67% (CI, 60-74) after transsphenoidal surgery(14).
References
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000 Oct;80(4):1523–631.
- Saleem M, Martin H, Coates P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin Biochem Rev. 2018 Feb;39(1):3–16.
- Gorvin CM. The prolactin receptor: Diverse and emerging roles in pathophysiology. J Clin Transl Endocrinol. 2015 May 16;2(3):85–91.
- Brooks CL. Molecular Mechanisms of Prolactin and Its Receptor. Endocr Rev. 2012 Aug;33(4):504–25.
- Liu X, Tang C, Wen G, Zhong C, Yang J, Zhu J, et al. The Mechanism and Pathways of Dopamine and Dopamine Agonists in Prolactinomas. Front Endocrinol. 2019 Jan 22;9:768–768.
- Shimazu S, Shimatsu A, Yamada S, Inoshita N, Nagamura Y, Usui T, et al. Resistance to dopamine agonists in prolactinoma is correlated with reduction of dopamine D2 receptor long isoform mRNA levels. Eur J Endocrinol. 2012 Mar;166(3):383–90.
- Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005;8(1):43–52.
- Vermeulen E, D’Haens J, Stadnik T, Unuane D, Barbe K, Van Velthoven V, et al. Predictors of dopamine agonist resistance in prolactinoma patients. BMC Endocr Disord. 2020 May 19;20(1):68.
- Almalki MH, Alzahrani S, Alshahrani F, Alsherbeni S, Almoharib O, Aljohani N, et al. Managing Prolactinomas during Pregnancy. Front Endocrinol. 2015;6:85.
- Huang HY, Lin SJ, Zhao WG, Wu ZB. Cabergoline versus bromocriptine for the treatment of giant prolactinomas: A quantitative and systematic review. Metab Brain Dis. 2018 Jun 1;33(3):969–76.
- Sabuncu T, Arikan E, Tasan E, Hatemi H. Comparison of the effects of cabergoline and bromocriptine on prolactin levels in hyperprolactinemic patients. Intern Med Tokyo Jpn. 2001 Sep;40(9):857–61.
- Brichta CM, Wurm M, Krebs A, Schwab KO, van der Werf-Grohmann N. Start low, go slowly – mental abnormalities in young prolactinoma patients under cabergoline therapy. J Pediatr Endocrinol Metab. 2019;32(9):969–77.
- Miura J, Kikuchi A, Fujii A, Tateishi T, Kaneko S. Pathological gambling associated with cabergoline in a case of recurrent depression. Drug Discov Ther. 2009 Aug;3(4):190–2.
- Zamanipoor Najafabadi AH, Zandbergen IM, de Vries F, Broersen LHA, van den Akker-van Marle ME, Pereira AM, et al. Surgery as a Viable Alternative First-Line Treatment for Prolactinoma Patients. A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2020 Mar 1;105(3):e32–41.
Kindly Let Us Know If This Was helpful? Thank You!


