An Overview of Indications of Humira
Humira, known generically as adalimumab, is a therapeutic drug used in managing a range of chronic inflammatory conditions. This includes diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn’s disease and ulcerative colitis, which are types of inflammatory bowel diseases. In addition, Humira is also indicated for plaque psoriasis, hidradenitis suppurativa, juvenile idiopathic arthritis, and certain forms of uveitis. This wide range of indications underscores the vital role of Humira in the management of debilitating inflammatory conditions.
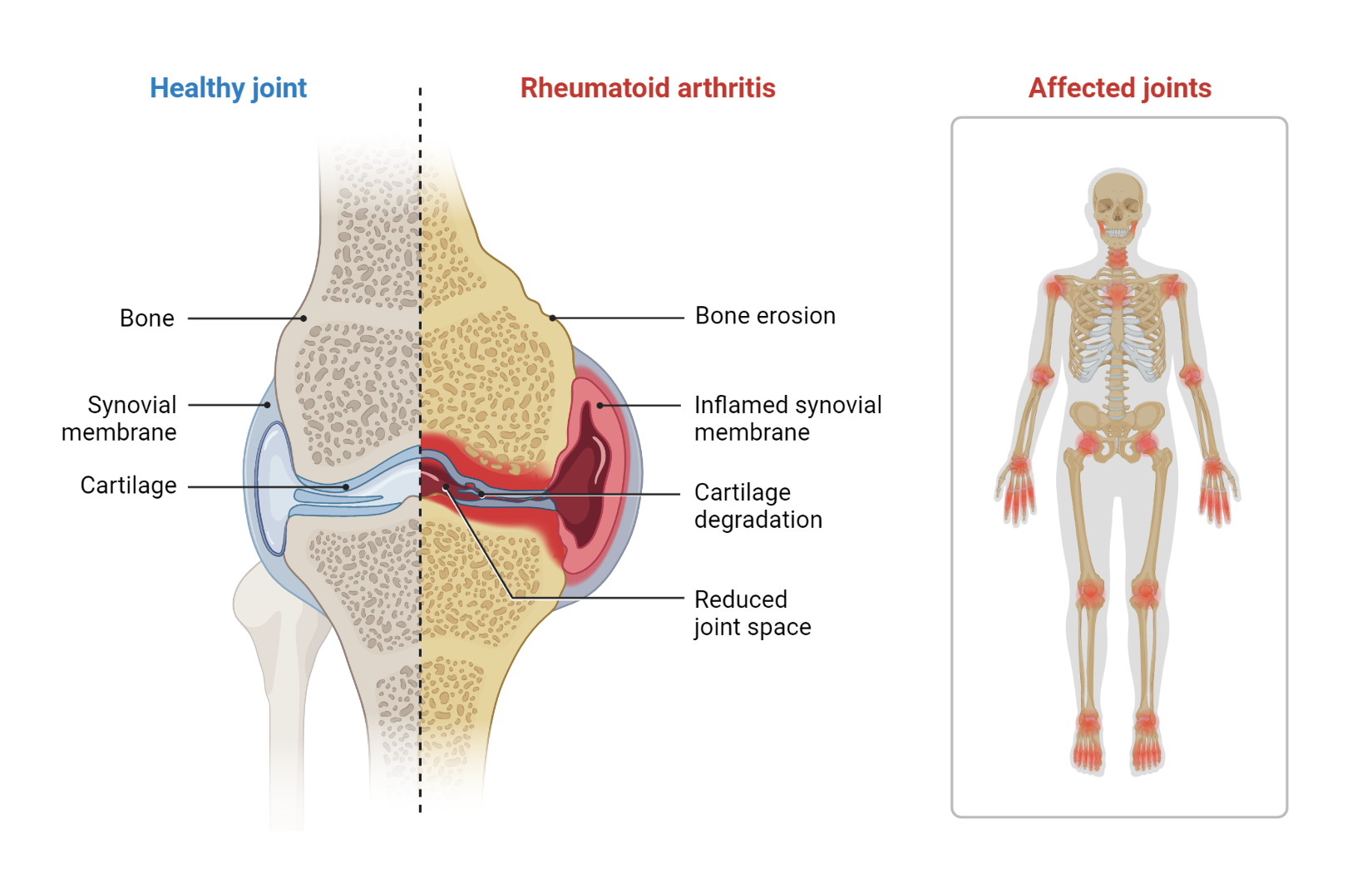
Pathophysiology of Rheumatoid Arthritis
The hallmark of this disease is inflammation of the synovium, the tissue that lines the joints, but it can also have wide-ranging systemic effects. Here, we break down the sequential steps in the pathogenesis of RA.
Step 1: Environmental Triggers and Genetic Factors
The development of RA is believed to be triggered by a combination of genetic and environmental factors. Certain genetic traits, such as specific HLA-DRB1 alleles, increase susceptibility to RA. On the environmental side, factors such as smoking or alterations in the gut microbiota are thought to be significant contributors. These factors lead to an initial immune response that breaks the body’s tolerance to its own tissues.
Step 2: Autoantibody Production
The immune system, due to this loss of tolerance, produces autoantibodies. These antibodies target proteins modified by a process called citrullination. These autoantibodies, known as Anti-Citrullinated Protein Antibodies (ACPAs), can be detected in patients’ blood often years before RA symptoms manifest.
Step 3: Activation of CD4+ T cells
CD4+ T cells, a type of immune cell, play a crucial role in the progression of RA. Antigen-presenting cells (APCs) activate CD4+ T cells in the joint synovium. Once activated, these T cells multiply and secrete pro-inflammatory cytokines such as TNF, IL-6, and IL-17, contributing to inflammation and recruitment of other immune cells to the synovium.
Step 4: Synovial Inflammation and Hyperplasia
Under the influence of inflammatory cytokines, synovial fibroblasts (cells that make up a portion of synovial tissue) are activated. These activated cells proliferate and form a tumor-like invasive tissue called pannus. The pannus contributes to joint damage by invading and eroding adjacent cartilage and bone.
Step 5: Matrix Degradation by MMPs
In the inflamed joint, synovial cells, chondrocytes, and other inflammatory cells produce enzymes known as matrix metalloproteinases (MMPs). These enzymes break down extracellular matrix proteins, leading to cartilage degradation, a major factor contributing to joint damage in RA.
Step 6: Bone Erosion by RANKL Action
The RANKL molecule (Receptor Activator of Nuclear Factor kappa-Β Ligand) is a primary driver of bone erosion in RA. RANKL promotes the differentiation and activation of osteoclasts, cells responsible for bone resorption. High levels of RANKL in the synovium of RA patients lead to increased osteoclast activity, contributing to bone erosion commonly seen in RA.
Step 7: Systemic Manifestations of RA
Lastly, RA is not just a disease of the joints. The inflammation also manifests itself systemically. Patients often experience systemic symptoms such as fatigue, fever, and depression. Chronic inflammation also accelerates atherosclerosis, increasing the risk of cardiovascular disease in RA patients.

Dissecting Humira’s Mechanism of Action
Humira belongs to a group of medicines known as TNF-alpha inhibitors. Its active ingredient, Adalimumab, is a specially designed monoclonal antibody that specifically targets and binds to tumor necrosis factor-alpha (TNF-α). TNF-α is a type of cytokine, a small protein released by immune cells, which plays a central role in mediating inflammatory responses in the body.

Overproduction or unregulated expression of TNF-α, as often observed in autoimmune diseases, leads to persistent inflammation, contributing to tissue damage and subsequent progression of the disease. Humira works by binding directly to TNF-α, effectively neutralizing its pro-inflammatory activity. Consequently, this reduces inflammation and alleviates symptoms associated with the conditions that Humira is designed to treat.
Administration and Dosage protocol for Humira
Humira is usually administered as a subcutaneous injection. Common sites for injection include the thigh or abdomen. The dose of Humira varies depending on the specific condition being treated and the individual patient’s response to therapy.
Dosage can range from 40mg administered every other week to an initial dose of 160mg, followed by 80mg two weeks later, and then maintenance doses administered every other week.
Potential adverse effects of Humira
As with all medications, Humira can cause side effects. Commonly reported adverse effects include reactions at the injection site, such as redness, rash, swelling, itching, or bruising. Other common side effects include headaches and rash.
However, more severe side effects can occur. These include serious infections (including tuberculosis, bacterial sepsis, and invasive fungal infections), the development of malignancies, and immune reactions (including a lupus-like syndrome).
Contraindications: When is Humira not suitable?
Humira is contraindicated in patients with a known hypersensitivity to Adalimumab or any of its excipients. It should not be initiated in patients with active infections, including clinically significant localized infections and active tuberculosis. Humira should also be used with caution in patients with a history of heart failure, demyelinating disease, or malignancies.
Monitoring Protocol
Patients receiving Humira should be closely monitored for any signs of infection due to the immunosuppressive nature of the drug. Regular screenings for latent tuberculosis and careful monitoring of injection sites for potential reactions are also recommended. Regular liver function tests may be considered, as there have been reports of acute liver injury with Humira. For patients with heart failure, routine clinical follow-ups are recommended to monitor the status of the disease.
Kindly Let Us Know If This Was helpful? Thank You!


