Physiology Of Aldosterone Action
Aldosterone stands out as the primary and most effective mineralocorticoid produced in humans. Other steroids might demonstrate a similar but typically weaker mineralocorticoid function. Both mineralocorticoids and glucocorticoids may show overlapping activities.
The fundamental functions of aldosterone are to:
- Preserve body sodium while disposing of potassium: Aldosterone enhances sodium reabsorption and simultaneously encourages potassium and hydrogen excretion through the walls of the epithelial cells in the kidney's distal convoluted tubule and collecting ducts. Consequently, water excretion reduces (due to passive tubular reabsorption), causing an increase in blood volume.
- Reduce the proportion of sodium to potassium ions in sweat and saliva: The transport of sodium into the cells (and the passive efflux of potassium) is heightened across the cell membranes of sweat and salivary gland ducts. In a warm environment where one might sweat excessively, the role of aldosterone becomes crucial to prevent an excessive loss of sodium through diaphoresis.
- Elevate the reabsorption of sodium from the colon and increase potassium excretion in feces. While aldosterone only controls the reabsorption of approximately 2% of the sodium filtered by the kidney, drugs inhibiting aldosterone's effects such as spironolactone are potent diuretics. These drugs can be beneficial in treating edema as they antagonize aldosterone's effect on sodium reabsorption in the kidney tubules, resulting in increased water excretion through urine.
renin angiotensin aldosterone cascade
What Is The Mechanism Of Action Of Aldosterone In Normal Physiology?
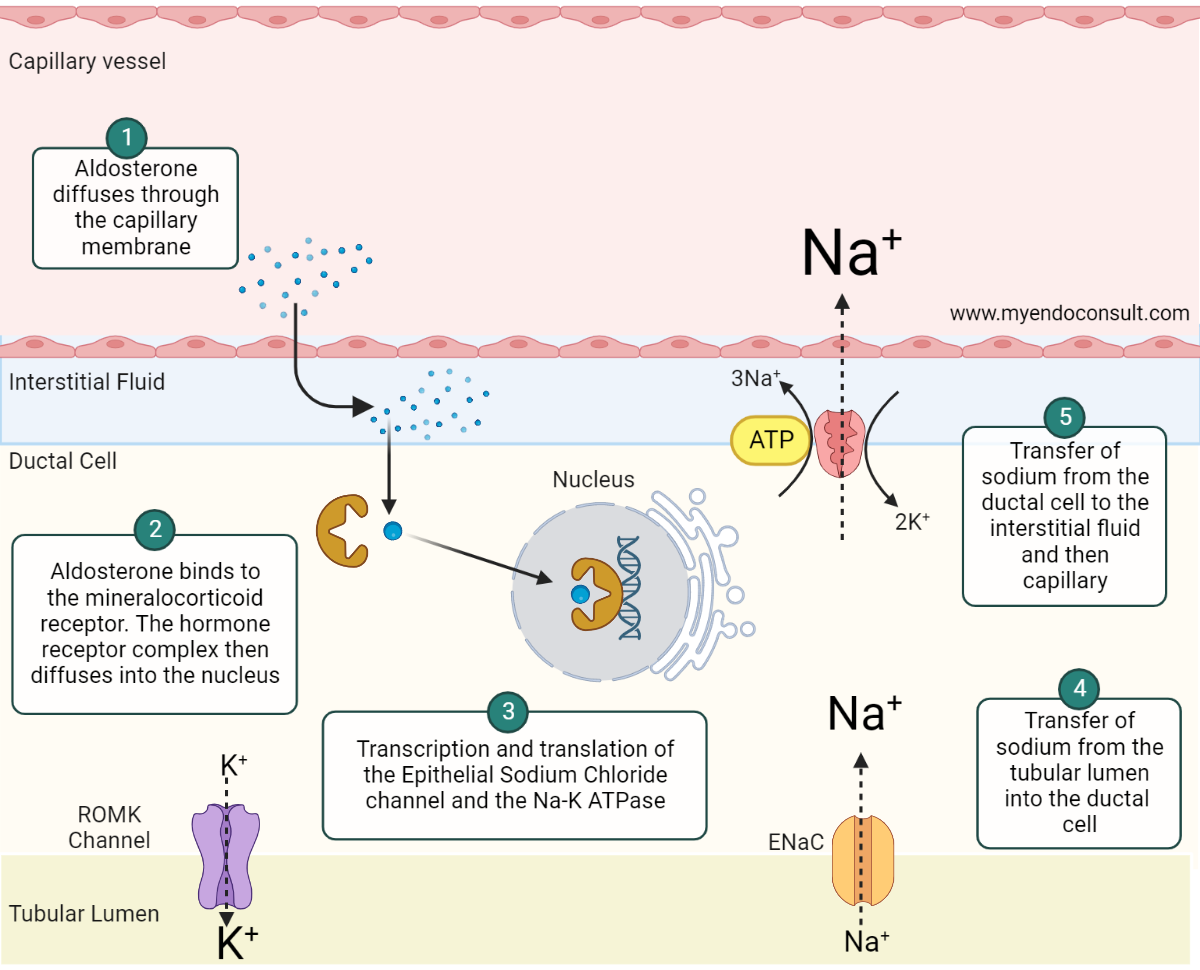
Aldosterone's impact on sodium reabsorption through epithelial cells typically takes one to two hours to initiate and occurs at a cellular level. This is facilitated by the interaction with a high-affinity cytosolic mineralocorticoid receptor, which shares a significant similarity with the glucocorticoid receptor. This receptor combines with nuclear DNA's regulatory components to prompt the creation of a sodium transport protein or channel. Increased production of Na+/K+-ATPase molecules, which transfer sodium from the tubular cells into the surrounding fluid, may also be triggered.
The mechanisms behind the effects on potassium and hydrogen excretion are less clear. The movement of potassium into the tubular fluid is probably a passive reaction to the amplified intracellular sodium load. Conversely, the exchange of hydrogen for sodium in the tubules is believed to occur due to a swift stimulation of a sodium-hydrogen antiporter in these cells.
The mechanism of action of aldosterone
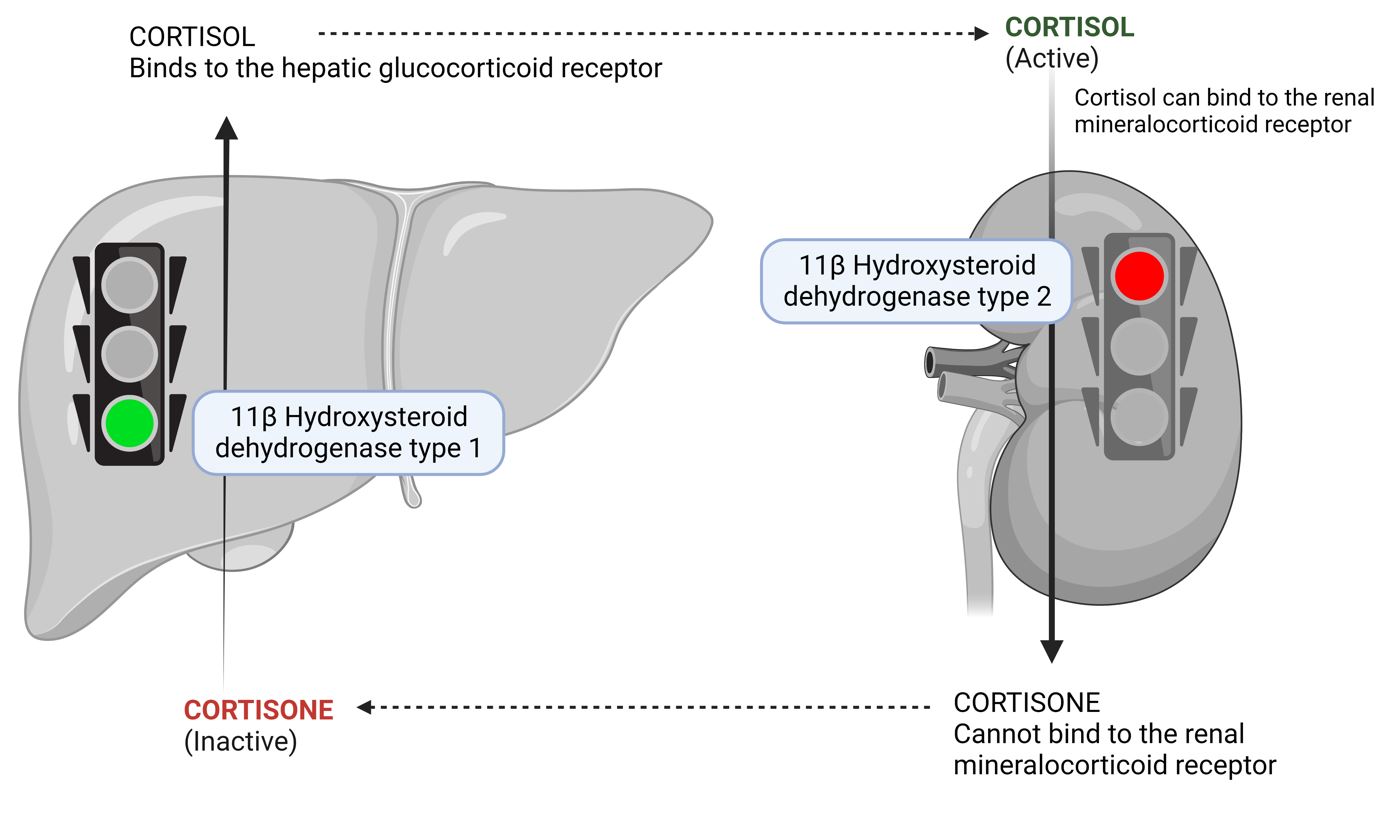
The 11-beta-hydroxysteroid dehydrogenase (11-HSD) in aldosterone-sensitive tissues contributes to the specificity of the intracellular mineralocorticoid receptor for mineralocorticoids. It does this by facilitating the transformation of circulating cortisol and corticosterone into their receptor-inactive analogs, cortisone and 11-dehydrocorticosterone, respectively.
It is worth noting that aldosterone itself is a poor substrate for 11-HSD. This effectively prevents glucocorticoids from reaching mineralocorticoid receptors, giving aldosterone its distinct role. In cases where 11-HSD is absent or its activity is diminished (such as due to excessive licorice consumption), the "protection" of the mineralocorticoid receptors is lost, which allows cortisol to exhibit mineralocorticoid effects in the kidneys. These individuals might exhibit symptoms of mineralocorticoid excess, such as sodium retention, hypertension, and lower plasma potassium levels (hypokalemia).
Two main categories of specific cell surface receptors for angiotensin II have been identified, known as AT1 and AT2, with AT1 having two further subtypes, AT1A and AT1B. The angiotensin II receptors in the adrenal cortex are primarily of the AT1B subtype, which upon activation, promotes the production of intracellular inositol trisphosphate (IP3), release of calcium from internal stores and subsequently stimulates the synthesis of aldosterone.
Regulation of Aldosterone
The regulation of aldosterone release is a complex process. While adrenocorticotropic hormone (ACTH) does stimulate some transient release of aldosterone and plays a role in maintaining the zona glomerulosa, it's not viewed as a significant contributor to the long-term management of aldosterone production and secretion. Instead, the renin-angiotensin system and the concentrations of sodium and potassium in the plasma primarily influence the release of aldosterone.
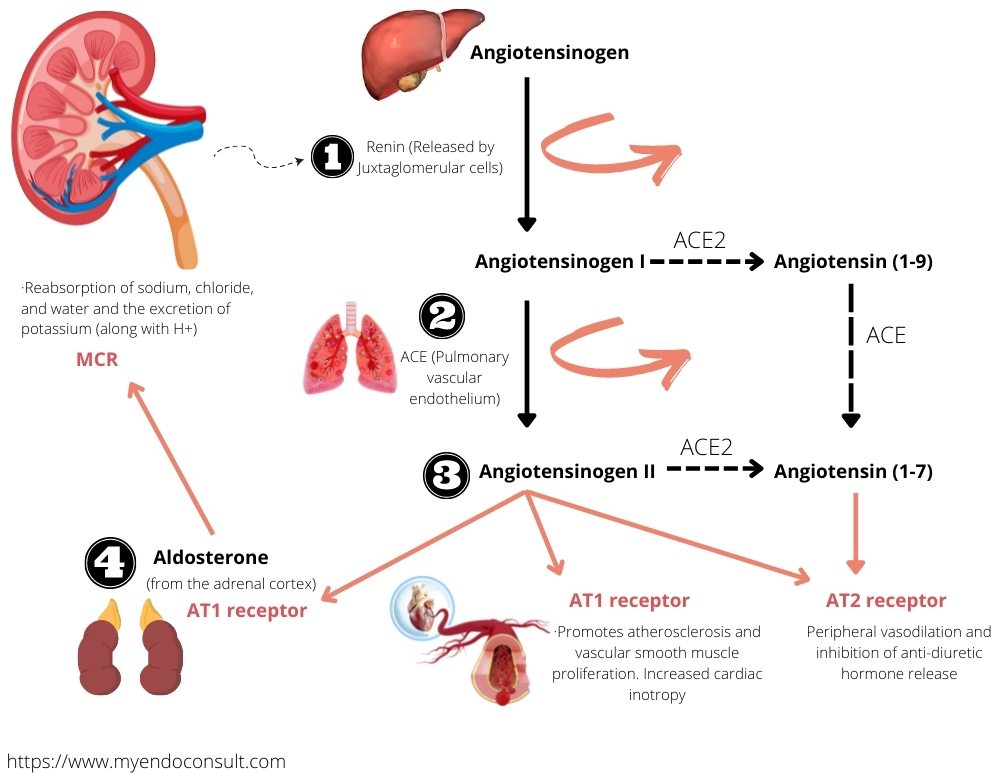
Renin is a proteolytic enzyme released by specialized cells located within the walls of the kidney's afferent arterioles, known as juxtaglomerular cells. This release happens when there is a decrease in renal blood pressure and a severe reduction in circulating blood volume, which can occur due to events such as hemorrhage or loss of salt or water.
Renin in the circulation promotes the formation of an inactive decapeptide, angiotensin I, from a precursor protein called angiotensinogen, which originates from the liver. Angiotensin I is quickly transformed in the lungs and plasma into the active octapeptide, angiotensin II, through the action of an enzyme known as angiotensin-converting enzyme (ACE). Angiotensin II directly stimulates the production and release of aldosterone from the adrenal cortex's zona glomerulosa.
Furthermore, angiotensin II has a strong vasoconstrictor effect, which helps maintain arterial blood pressure under extreme conditions. It also influences water intake via the subfornical organ in the brain, a phenomenon known as the dipsogenic effect.
Circulating angiotensin II is broken down by enzymes such as glutamyl aminopeptidase A into angiotensin III and, ultimately, angiotensin IV. These molecules, though less potent, share similar vasoconstrictor properties and the ability to stimulate aldosterone release with angiotensin II.
Types of Hyperaldosteronism
Hyperaldosteronism, can be categorized into two types: primary and secondary hyperaldosteronism.
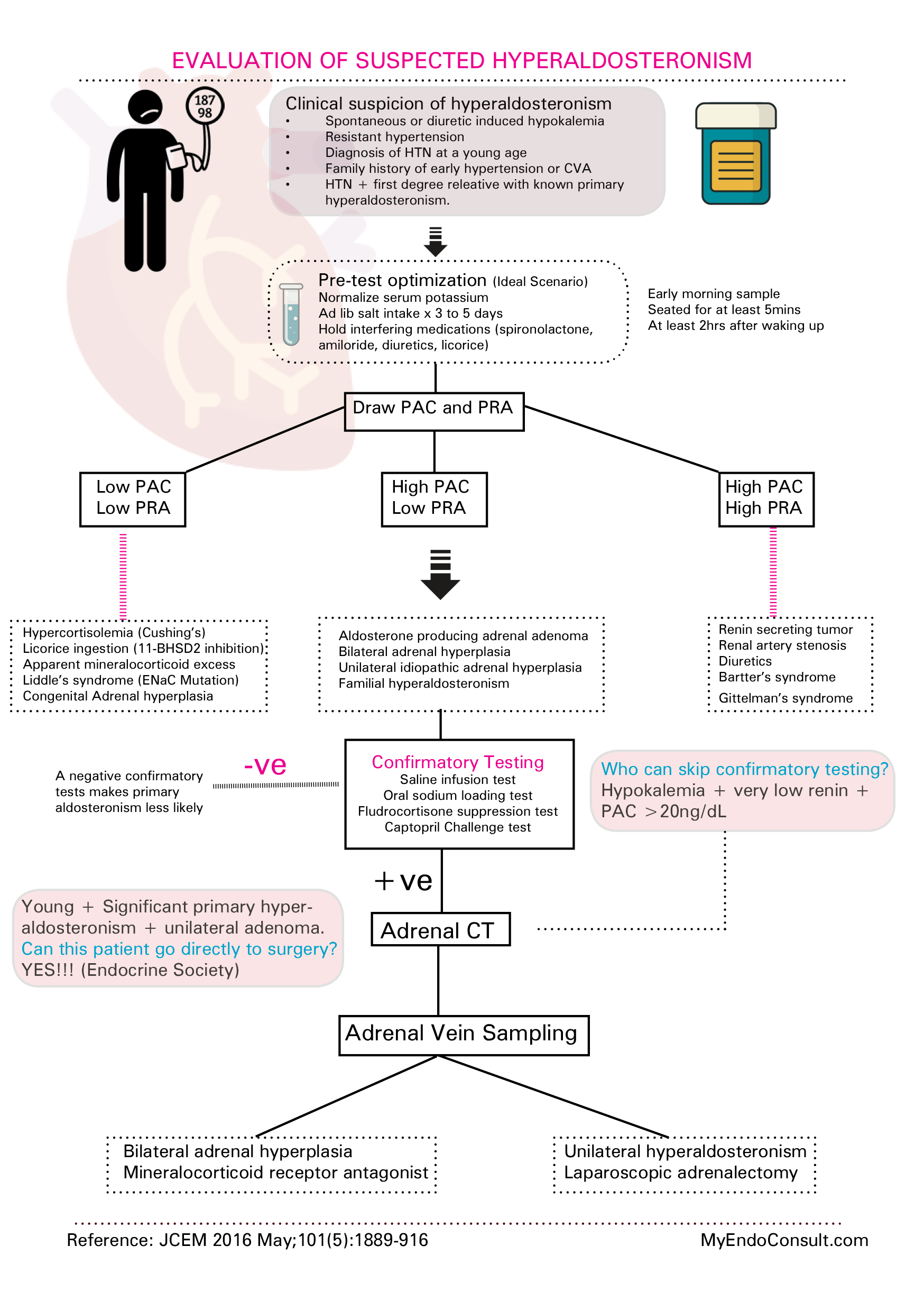
Primary hyperaldosteronism, also known as Conn's Syndrome, typically arises due to bilateral adrenal hyperplasia or a small tumor, referred to as an adenoma, in the adrenal zona glomerulosa. Patients affected by this condition may experience hypertension due to sodium and water retention (although without peripheral edema), and hypokalemia.
Symptoms can include muscle weakness, fatigue, and arrhythmias. Mild metabolic alkalosis may also be present. In this condition, plasma renin levels are usually low. Diagnosis is established through the detection of elevated aldosterone levels in plasma or urine combined with a low plasma renin level.
In these patients, saline loading to expand blood volume would not suppress the high aldosterone levels. Treatment generally includes surgical removal of the tumor causing the condition or long-term use of an aldosterone receptor antagonist such as spironolactone, which could also be employed to manage patients preoperatively.
Secondary Aldosteronism occurs due to an abnormal increase in renin release, leading to higher angiotensin II levels. These patients may present with peripheral edema.
The condition can be caused by various factors, such as poor blood flow to the kidneys (as in renal artery stenosis), severe high blood pressure associated with progressive kidney failure due to damage of the small arteries in the kidney (malignant hypertension), a tumor in the juxtaglomerular cells of the kidney, excessive loss of sodium and water during diuretic therapy or dietary sodium restriction, or conditions like congestive heart failure or liver cirrhosis.
The treatment approach focuses on addressing the root cause of the abnormal activation of the renin-angiotensin system, along with the long-term administration of spironolactone.
Workup for Hyperaldosteronism (Infographic)
Evaluation of hyperaldosteronism
Kindly Let Us Know If This Was helpful? Thank You!


