Embryonic Development and Maturation of the Adrenal Gland
During the sixth week of embryonic development, the adrenal glands begin to form, and they arise from clusters of mesenchymal cells adjacent to the urogenital ridge and along the wall of the celomic cavity. Throughout fetal growth and up to the first year after birth, the adrenal cortex is characterized by an inner fetal zone and an outer permanent zone.
Layers of the adrenal gland
The inner fetal zone eventually regresses, while the permanent zone proliferates and differentiates into the outer glomerulosa and inner fasciculata zones. Subsequently, a more inner zona reticularis develops.
Anatomy of the Adult Adrenal Gland
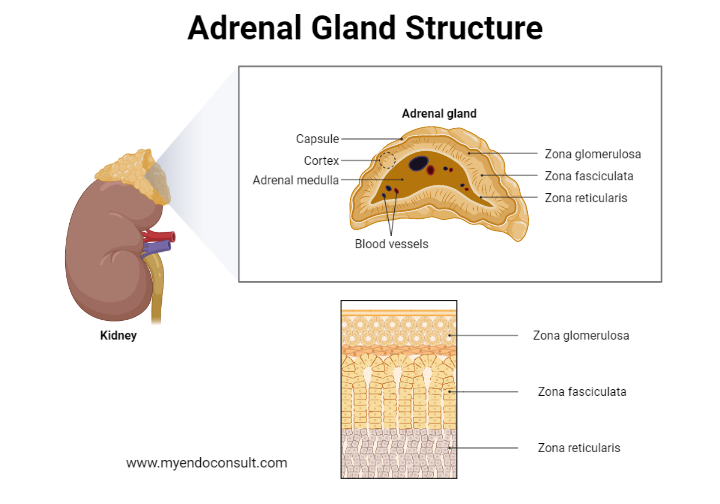
In adults, the adrenal gland comprises a cortex containing an outer zona glomerulosa, a middle zona fasciculata, and an inner zona reticularis, which encloses the adrenal medulla. The zona fasciculata can be distinguished from the outer glomerulosa and inner reticularis cells due to its cellular arrangement resembling bundled chains. The adrenal medulla houses chromaffin cells, which are easily stained with chromic acid salts.
Each pyramid-shaped (left) or crescent-shaped (right) adrenal gland is encapsulated and connected to the upper pole of its respective kidney. In adults, adrenal glands weigh approximately 5 grams and measure roughly 5x2 cm in length and 1 cm in thickness.
Vascular supply of the adrenal glands
Arterial Supply to the Adrenal Glands
The adrenal glands receive blood from various sources, including the abdominal aorta, inferior phrenic, renal, and intercostal arteries. Small arterial branches from these sources supply each adrenal gland, forming an arteriolar network just beneath the gland's capsule. These arteries are:
Superior adrenal (suprarenal) arteries arise from the inferior phrenic arteries, which branch off the abdominal aorta and typically supply the superior portion of the adrenal glands.
Middle adrenal (suprarenal) arteries originate directly from the abdominal aorta, supplying the central part of the adrenal glands.
Inferior adrenal (suprarenal) arteries stem from the renal arteries and provide blood to the inferior portion of the adrenal glands.
Blood Flow within the Adrenal Glands
Once the arterial blood enters the adrenal gland, it flows through an arteriolar network located just below the capsule. From there, it descends through radial capillaries that traverse the three cortical zones (zona glomerulosa, zona fasciculata, and zona reticularis) before reaching the adrenal medulla. This vascular arrangement ensures that the adrenal cortex receives blood before the medulla, allowing for efficient oxygen and nutrient exchange.
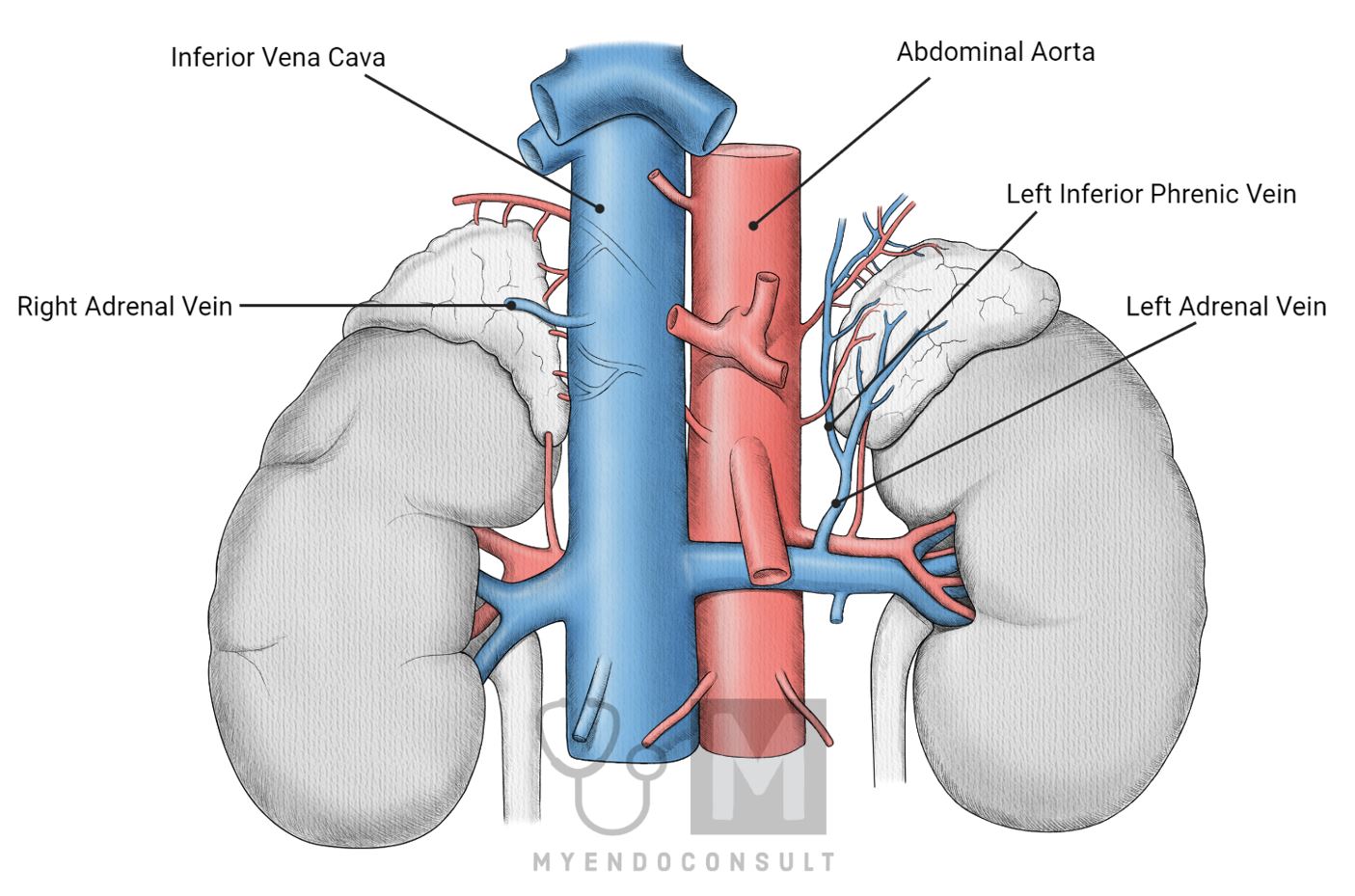
Venous Drainage of the Adrenal Glands
Blood from the adrenal glands drains into the central vein within the medulla, which then exits through the right and left adrenal veins. These veins follow different courses:
Right adrenal vein: This short vein drains directly into the inferior vena cava, which carries blood back to the heart.
Left adrenal vein: This longer vein typically drains into the left renal vein or occasionally into the left inferior phrenic vein before entering the inferior vena cava.
Adrenal Medulla Blood Supply
The majority of arterial blood (over 90%) reaches the adrenal medulla indirectly after passing through the three cortical zones, becoming partially deoxygenated by the time it reaches the chromaffin cells. However, a small portion of the medullary arterial blood (less than 10%) reaches the chromaffin cells directly through small arterioles that penetrate the cortex. This direct supply ensures that the adrenal medulla receives adequate oxygen and nutrients to support its essential functions, such as producing catecholamines (epinephrine and norepinephrine).
Adrenocortical Hormones: Production, Storage, Release, and Transport
The adrenal cortex is divided into three distinct layers, or zones. The outer 15% of the cortex is the zona glomerulosa, while the main part (around 75%) consists of radial rows of cells forming the zona fasciculata. Glomerulosa cells are small and arranged in spherical clusters, while fasciculata cells are larger and lipid-filled, extending towards the irregularly distributed reticularis cells containing minimal lipid. The medulla's chromaffin cells are distinct and separate, serving as typical secretory cells with numerous granules.
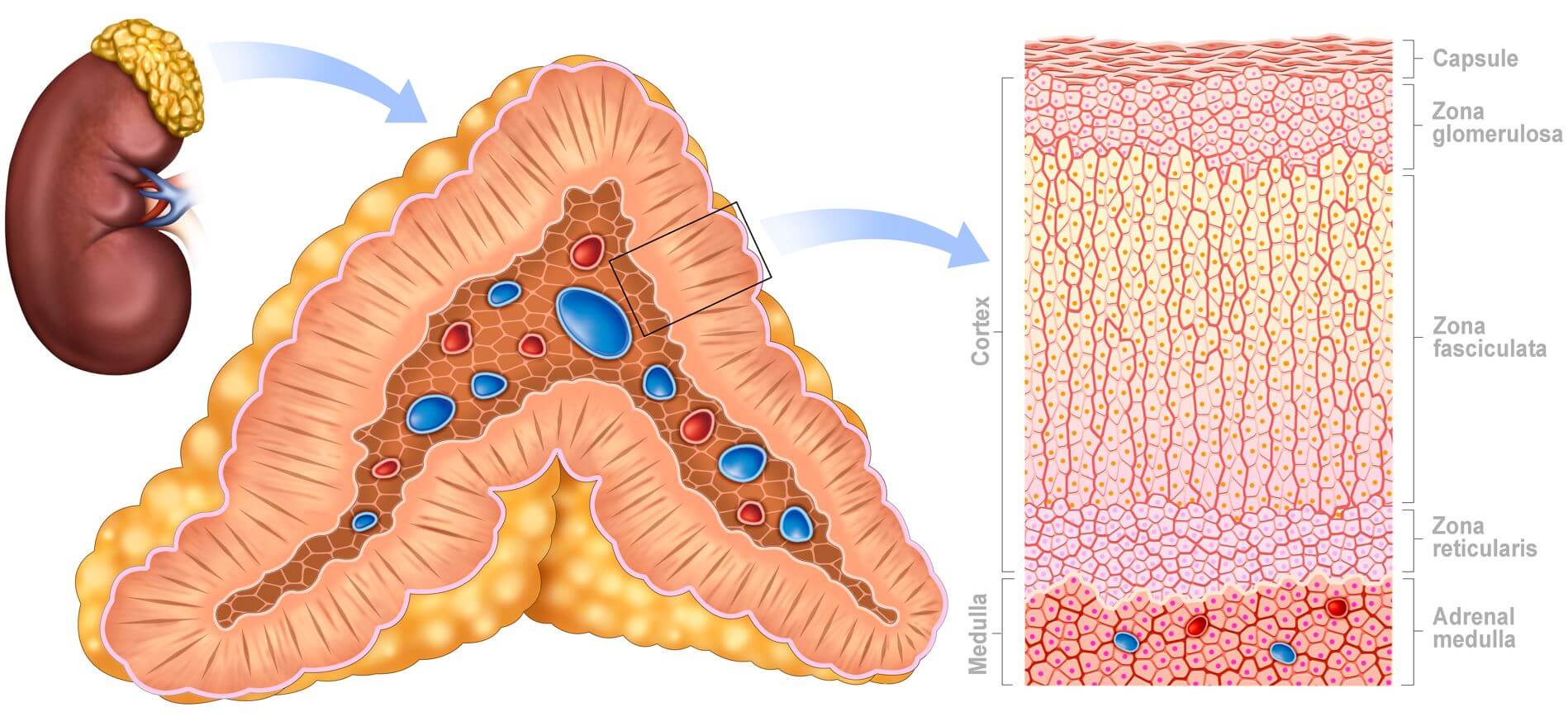
Zones of the adrenal glands
The three cortical zones synthesize steroid hormones known as corticosteroids. Since adrenocortical hormones cannot be stored extensively due to their ability to cross lipid membranes and enter the general circulation, they are primarily synthesized "on demand" when adrenocortical cells are appropriately stimulated. Nevertheless, a small amount of cholesterol is present (stored) in the cells in an esterified form, readily available for hormone synthesis.
Corticosteroids are traditionally categorized based on their primary physiological activities:
- Mineralocorticoids, which play a crucial role in mineral regulation (Na+, K+ ions)
- Glucocorticoids, which significantly influence metabolic regulation
- Sex hormones, such as androgens and estrogens, which are typically associated with the gonads.
Corticosteroid synthesis involves cell-dependent, enzyme-induced conversions of substrates to end products via various intermediates. The initial precursor molecule is cholesterol, which primarily reaches the cells as low-density lipoprotein (LDL) cholesterol or is synthesized from acetyl coenzyme A within the cells. The first critical step in corticosteroid hormone synthesis is the rate-limiting transport of intracellular cholesterol from the outer to the inner mitochondrial membrane, where it transforms into pregnenolone.
The unique enzymes present in each adrenal cortex zone's cells facilitate the production of specific hormones. In humans, the primary mineralocorticoid produced by the zona glomerulosa is aldosterone, while the primary glucocorticoid is cortisol (or hydrocortisone). The weak androgens produced are primarily dehydroepiandrosterone (DHEA) and androstenedione, which can be converted to more potent androgens or estrogens in peripheral tissues, depending on the presence of reductase or aromatase enzymes in the cells.
Regulation of Adrenocortical Hormone Secretion
Hormone secretion in the adrenal cortex is regulated by a complex interplay of hormonal and neural signals. The hypothalamic-pituitary-adrenal (HPA) axis plays a central role in the regulation of cortisol production. The hypothalamus secretes corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary gland to produce adrenocorticotropic hormone (ACTH). In turn, ACTH prompts the adrenal cortex to synthesize and release cortisol. The cortisol levels in the bloodstream provide negative feedback to the hypothalamus and pituitary gland, ensuring homeostatic control of cortisol secretion.
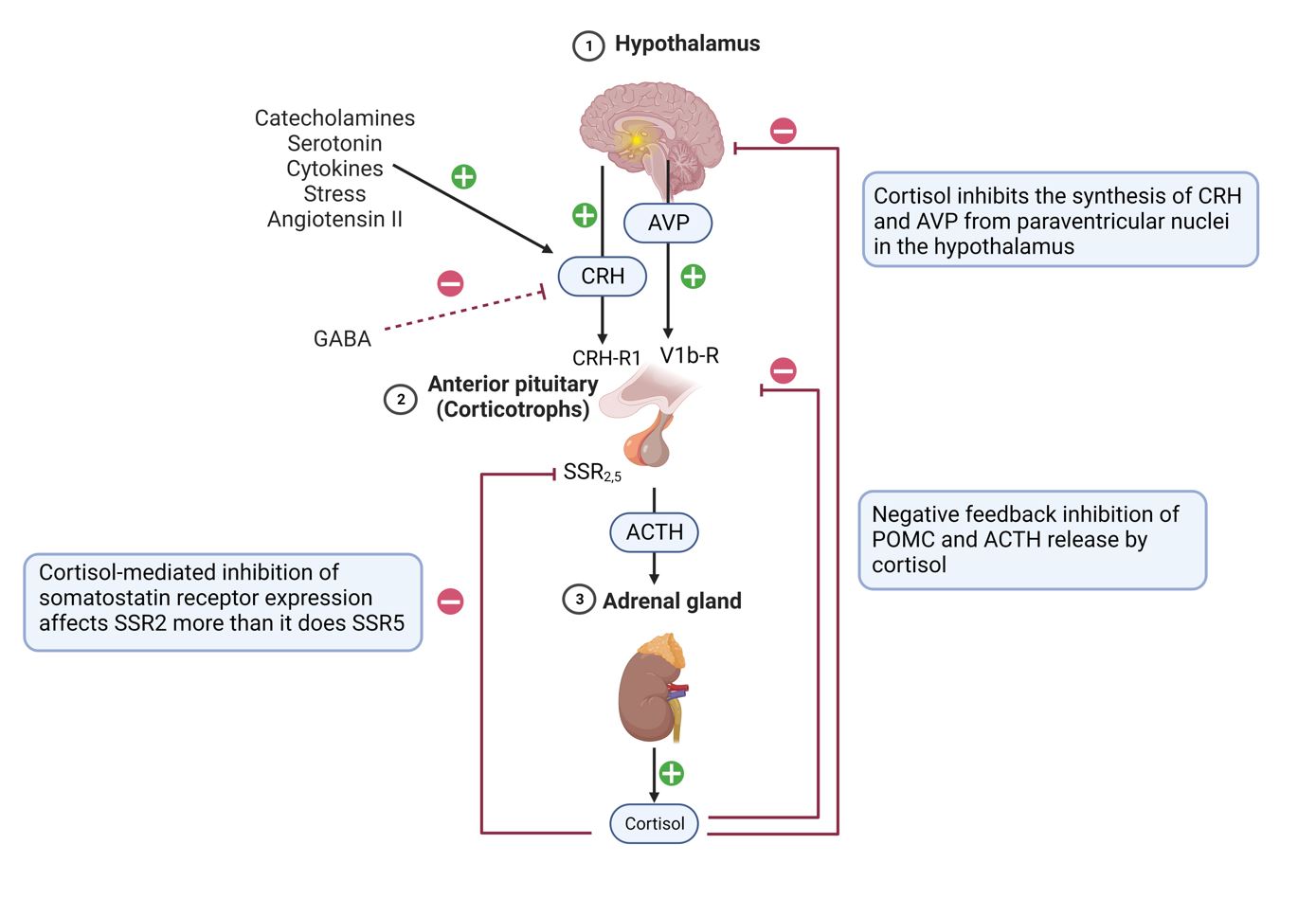
Aldosterone secretion in the zona glomerulosa is primarily regulated by the renin-angiotensin-aldosterone system (RAAS). Renin, released by the kidneys in response to low blood pressure or decreased blood flow, catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin-converting enzyme (ACE) then converts angiotensin I to angiotensin II, which stimulates aldosterone production in the adrenal cortex. Increased aldosterone levels promote sodium reabsorption and potassium excretion in the kidneys, ultimately increasing blood volume and blood pressure.
Adrenal Androgens and Sex Hormone Regulation
Multiple factors, including ACTH and other growth factors, cytokines, and hormones regulate androgen synthesis and secretion. The release of adrenal androgens, such as DHEA and androstenedione, is primarily controlled by ACTH, which stimulates their synthesis in the zona reticularis. The adrenal androgens can be converted to more potent sex hormones, including testosterone and estradiol, in peripheral tissues. The gonads also produce these hormones, and their levels are regulated through feedback mechanisms involving the hypothalamus and pituitary gland.
Hormone Production in Different Adrenal Cortex Zones
Zona Glomerulosa: Mineralocorticoids
In the zona glomerulosa, progesterone is converted to deoxycorticosterone by 21-hydroxylase, which is found in the microsomes and endoplasmic reticulum. Deoxycorticosterone is then converted to corticosterone by the mitochondrial enzyme 11β-hydroxylase. In humans, corticosterone, a weak mineralocorticoid, is converted to the far more potent molecule aldosterone via an intermediate molecule, 18-hydroxycorticosterone, by aldosterone synthase.
Zona Fasciculata and Zona Reticularis: Glucocorticoids and Androgens
The fasciculata and reticularis cells do not contain aldosterone synthase and thus cannot produce aldosterone. However, they do contain 17α-hydroxylase, which is absent from the glomerulosa cells. This enzyme, located in the microsomal-endoplasmic reticulum fraction, converts both pregnenolone and progesterone to their 17α-hydroxy metabolites, respectively.
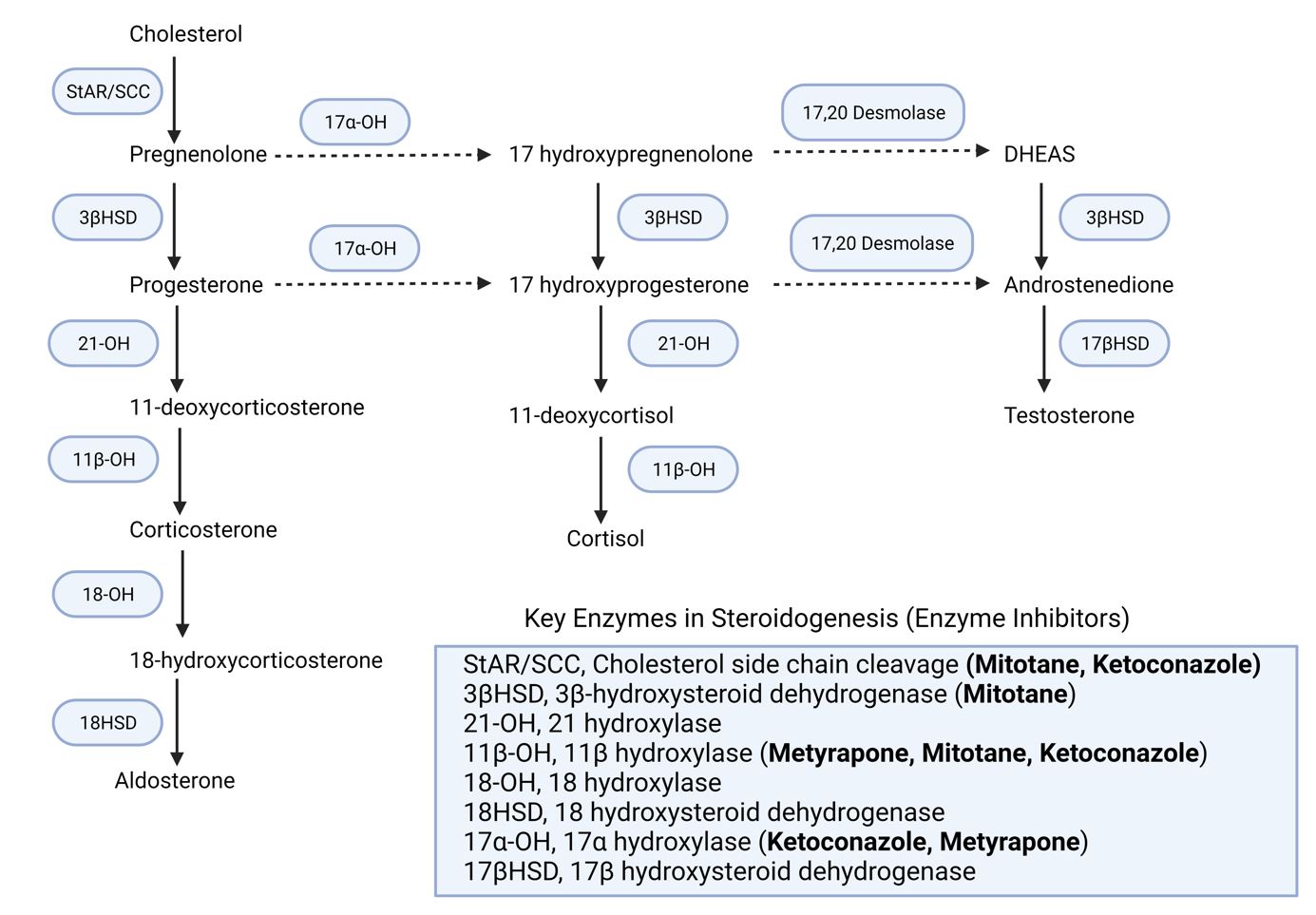
Mainly in the fasciculata cells, but also to some extent in the reticularis cells, the 17α-hydroxyprogesterone is converted to 11-deoxycortisol by the enzyme 21-hydroxylase, and this is then converted by 11β-hydroxylase to the final end product in humans, the powerful glucocorticoid cortisol. Both 17α-hydroxypregnenolone and 17α-hydroxyprogesterone can also be converted to the weak androgens dehydroepiandrosterone (DHEA) and androstenedione, respectively, by the action of 17α-hydroxylase, mainly in the reticularis cells but also to some extent in fasciculata cells.
Weak androgens, primarily DHEA and androstenedione, can be converted to more potent androgens or estrogens in peripheral tissues, depending on the presence of reductase or aromatase enzymes in the cells. Adrenal androgens account for around 50% of circulating androgens in females, with the remaining portion coming from thecal cells of developing ovarian follicles and, later in the menstrual cycle, the corpus luteum. In males, adrenal androgens are less significant since the majority of circulating androgen content comes from testosterone (and dihydrotestosterone) produced by the testes.
Kindly Let Us Know If This Was helpful? Thank You!


