Overview of hypercalcemia of Malignancy
Hypercalcemia, characterized by abnormally high levels of calcium in the blood, is a condition often associated with certain malignancies. However, it is important to note that not all cancers showing bone metastases will present with hypercalcemia. For example, tumors related to the gastrointestinal system and the female reproductive system, despite often developing bone metastases, rarely exhibit hypercalcemia.
Malignancies more commonly associated with hypercalcemia include squamous cell carcinomas, specifically those affecting the lung, head, and neck; various forms of breast cancer; hematologic cancers such as myeloma and T cell lymphomas; renal cancer; and less common cancers such as VIPomas and cholangiocarcinomas. It is interesting to note that, unlike many other hormone-related syndromes, oat cell carcinoma of the lung rarely triggers hypercalcemia.
Although hypercalcemia is a frequent occurrence in prevalent cancers such as lung and breast cancer, there are also widespread cancer types in which hypercalcemia is rarely observed.
What are the mediators of calcium regulation?
Hypercalcemia can arise from various conditions, including hyperparathyroidism, malignancies, and certain types of medications. The regulation of calcium balance in the body is a complex process involving several key players, with renal 1α-hydroxylase acting as one of the major mediators.
Renal 1α-Hydroxylase and Its Role in Calcium Regulation
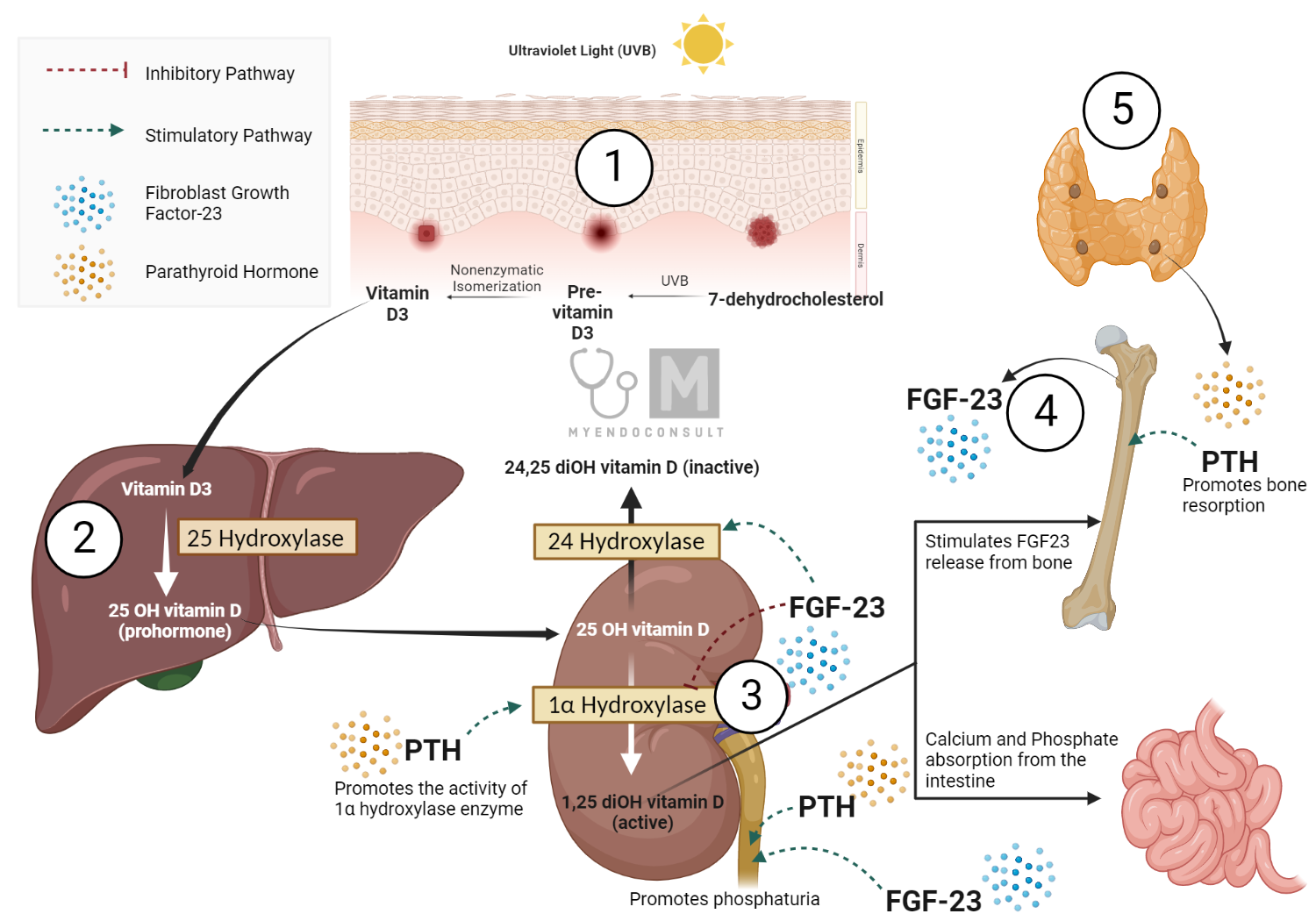
The 1α-hydroxylase enzyme, expressed in the proximal convoluted tubule of the kidney, plays a critical role in maintaining calcium homeostasis. It catalyzes the conversion of 25-hydroxyvitamin D (25(OH)D), an inactive form of vitamin D, into its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D or calcitriol).
Renal 1α-hydroxylase activity is modulated by various factors. It is stimulated by parathyroid hormone (PTH), hypophosphatemia, hypocalcemia, growth hormone (GH), estrogen, and prolactin. On the other hand, it is inhibited by fibroblast growth factor 23 (FGF23), 1,25(OH)2D itself, hypercalcemia, hyperphosphatemia, and certain drugs, including glucocorticoids and ketoconazole.
Parathyroid Hormone (PTH)
PTH, produced by the parathyroid glands’ chief cells, is a primary regulator of calcium homeostasis. When calcium levels fall below a certain threshold, PTH release is stimulated. PTH interacts with receptors on osteoblasts, resulting in cytokine release, which, in turn, triggers osteoclast activity. This cascade of events leads to the release of calcium and phosphate from bone.
Also, in the distal convoluted tubule (DCT) of the kidney, PTH increases calcium reabsorption and decreases phosphate reabsorption. PTH stimulates the activity of 1α-hydroxylase, resulting in the conversion of 25-OH vitamin D3 (calcidiol) to its active form, calcitriol.
Table 1. Comparison of intact PTH and PTHrp peptide hormones
| Biochemical feature | PTH | PTHrp |
| Structure | 84 amino acids | 139-173 amino acids |
| Target receptor(s) | PTH/PTHrp | PTH/PTHrp |
| Time of expression | Postnatal life | Expressed only during intrauterine life |
| Effect on 1α-hydroxylase | Stimulates enzyme activity | No effect |
| Renal calcium handling | Increased calcium reabsorption | Increased calcium reabsorption |
| Actions | Endocrine | Endocrine, autocrine or paracrine activity. |
| Physiologic effect | Bone remodeling | Transplacental calcium transfer. Bone formation during intra-uterine life |
Vitamin D
Vitamin D is a prohormone produced in the skin via the action of sunlight on 7-dehydrocholesterol, forming vitamin D3 (cholecalciferol). The conversion of cholecalciferol to its active form, calcitriol, is facilitated by renal 1α-hydroxylase, thereby enhancing calcium and phosphate absorption from the gut.
Calcitonin
Calcitonin is secreted by the parafollicular C cells of the thyroid gland. This secretion occurs when extracellular calcium levels rise above a certain threshold. Calcitonin acts primarily to suppress osteoclastic activity, thereby reducing bone resorption and subsequently lowering serum calcium levels.
Fibroblast Growth Factor 23 (FGF-23)
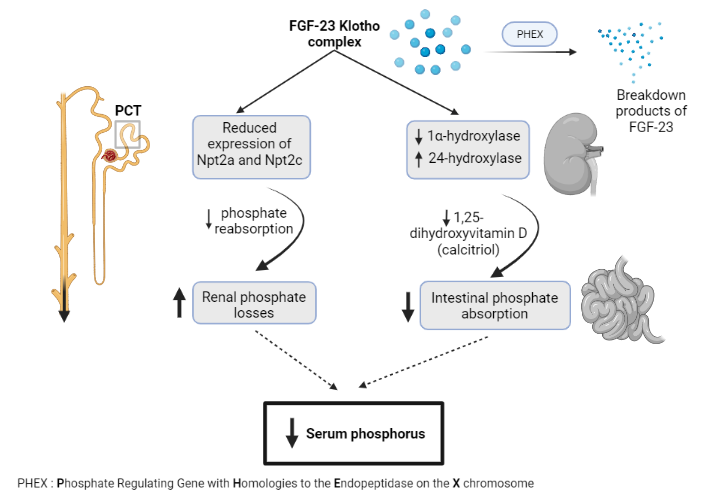
FGF-23 is primarily expressed in bone and connective tissues. It inhibits renal tubular phosphate transport, mainly in the proximal convoluted tubule, by suppressing the translocation of intracellular sodium-phosphorus co-transporters (NaPi-2a and 2c) to the cell membrane. This inhibition results in phosphaturia, the excretion of phosphate in urine. FGF-23 also inhibits renal 1α-hydroxylase activity, reducing the conversion of calcidiol to calcitriol and thus decreasing intestinal phosphate reabsorption.
Bone resorption
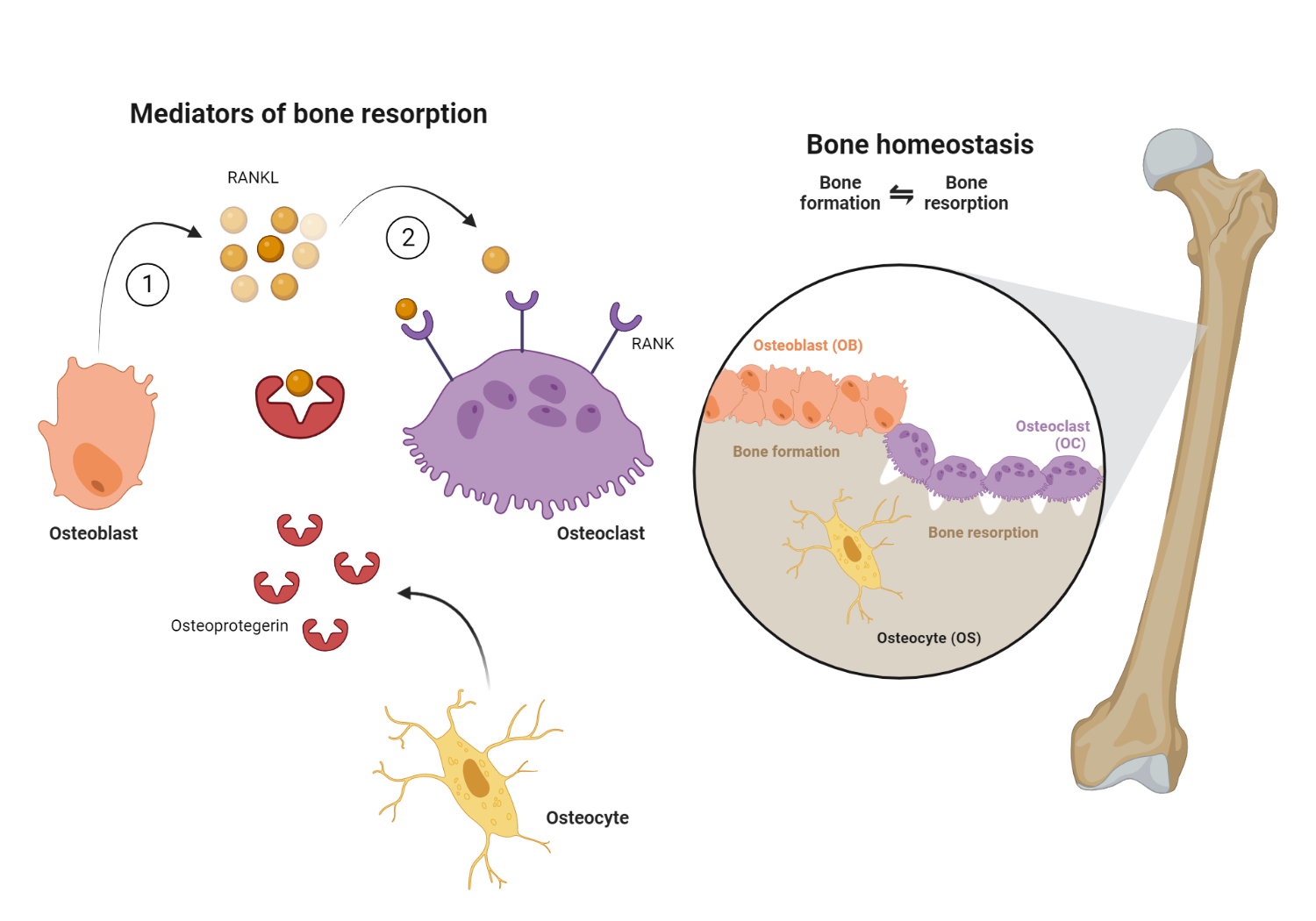
The dynamic interplay between osteoblasts and osteoclasts is central to bone remodeling and calcium homeostasis. Osteoblasts are bone-building cells, whereas osteoclasts are bone-resorbing cells.
Osteoclastic activity is primarily triggered by the receptor activator of nuclear factor kappa-B ligand (RANKL). This protein, present on the surface of osteoblasts, interacts with its receptor, RANK, on osteoclasts. The interaction stimulates osteoclasts to increase bone resorption, releasing calcium into the bloodstream.
What is the pathophysiology of hypercalcemia of malignancy?
Hypercalcemia is a complex occurrence influenced by the interplay of various pathological processes that impact the movement of calcium across the skeleton, kidneys, and gut. Primarily, this condition arises from excessive release of calcium from the bone into the extracellular fluid due to increased bone breakdown by osteoclasts.
Table 2. Mechanism of various forms of hypercalcemia of malignancy
| Type of Tumor(s) | Mediator of hypercalcemia | Mechanism |
| Breast cancer, Ovarian/Endometrial cancer, Renal/Urothelial cancer, Squamous cell carcinoma (lung, head, neck and esophagus) | PTHrp production | Activation of the PTH receptor by PTHrp, leading to excessive bone resorption. |
| Lymphoma | 1,25-dihydroxyvitamin D (1,25(OH)2D or calcitriol) | Increased absorption of intestinal calcium |
| Bone metastases (possible primaries including breast cancer, multiple myeloma and lymphomas) | Local factors (mainly cytokines) | Increased bone resorption |
| Rare solid tumors (evidence from few case reports) | Ectopic production of intact PTH | Activation of the PTH receptor by PTH, leading to excessive bone resorption. |
However, it is equally crucial to note the role of renal factors. Generally, an increase in bone calcium influx is counterbalanced by its enhanced renal excretion, thereby maintaining the balance of extracellular fluid calcium. However, in malignancies, this balance can be disrupted due to several renal events, including volume depletion leading to impaired glomerular filtration and consequent increased reabsorption of sodium and calcium in the proximal tubules. In addition, an increase in renal calcium conservation can occur, irrespective of volume depletion.
Clinical studies consistently reveal that patients with malignancy-related hypercalcemia exhibit increased renal tubular re-absorption of calcium, regardless of whether it is due to humoral mechanisms or local osteolytic bone disease*. The underlying mechanism is uncertain, but it might be related to the overproduction of parathyroid hormone-related protein (PTHrP) in many patients. However, in some cases, such as myeloma, hypercalcemia develops without increased PTHrP levels, and is rather due to the production of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage inflammatory protein-1α.
The importance of these kidney effects can vary depending on the type of tumor. For example, in myeloma, hypercalcemia rarely occurs unless glomerular filtration is compromised. On the other hand, in solid tumors associated with hypercalcemia, glomerular filtration typically remains adequate, and increased renal tubular calcium reabsorption is mostly due to the impact of tumor-derived systemic factors such as PTHrP.
Table 3. Comparison of humoral hypercalcemia of malignancy (HHM) and osteolysis-mediated processes
| Clinical feature | HHM | Osteolysis |
| Prevalence in hypercalcemia of malignancy | 80% | 20% |
| Mechanism | Circulating PTHrp | Cytokines and local PTHrp |
| Calcium and Phosphate | High calcium, low phosphate | High calcium, normal phosphate |
| Intact PTH | Low to undetectable | Low to undetectable |
| Type of malignancy | Squamous cell carcinoma | Multiple myeloma and lymphoma. |
Hematological Malignancies associated with hypercalcemia
Myeloma
In myeloma, a cancer that forms in a type of white blood cell called a plasma cell, osteolytic bone lesions are nearly always present, and 20% to 40% of patients experience hypercalcemia. However, hypercalcemia is rare unless the patient has compromised glomerular filtration. Patients with myeloma are prone to renal failure due to factors such as Bence Jones proteinuria, urinary tract infection, and uric acid nephropathy. The destruction of bone is attributed to local osteotropic cytokines produced by myeloma cells. The release of these factors activates nearby osteoclasts, which then resorb the bone, leading to characteristic bone lesions.
In myeloma, one of the major factors driving increased osteoclast activity may be RANKL, an important mediator of bone resorption.
Lymphoma
Certain types of lymphomas are also linked with bone lesions and hypercalcemia. In numerous instances, lymphoma cells have the ability to produce PTHrP. Patients with these tumors have elevated serum PTHrP levels and other characteristics of humoral hypercalcemia of malignancy (HHM) syndrome. There is also evidence to suggest that some tumors can synthesize 1,25-dihydroxyvitamin D, a potent bone resorbing factor. This could contribute to the osteolytic bone lesions seen in this condition. Some patients also have elevated serum 1,25(OH)2D levels.
Metastatic Solid Tumors
Hypercalcemia is a common condition in patients with extensive bone destruction due to osteolytic bone metastases. Although many types of tumors can cause hypercalcemia in tandem with bone metastasis, the condition is most frequently related to breast cancer, further complicated by extensive bone metastases. It is noted that hypercalcemia seldom arises early in the course of the disease or without extensive bone metastasis.
The primary mechanism for bone destruction is likely an increase in osteoclast activity. Also, tumor cells could directly destroy bone independent of osteoclasts. However, the evidence leans toward osteoclastic bone resorption as the main mechanism.
In part, the affinity of tumor cells for bone could be due to the fact that bone serves as a substantial storage area for growth regulatory factors. Bone is particularly rich in transforming growth factor beta (TGF beta), among other growth regulatory factors, such as bone morphogenetic proteins, heparin-binding fibroblast growth factors, platelet-derived growth factors, and insulin-like growth factors I and II. These factors may be released locally when bone resorption occurs. This phenomenon has been most notably demonstrated with TGF-beta, which becomes active when bone is resorbed. TGF-beta can influence the behavior of numerous tumor cells, especially breast cancer cells, potentially increasing the production of parathyroid hormone-related protein (PTHrP).
Non-metastatic Solid Tumors associated with hypercalcemia
In certain cases, hypercalcemia occurs despite the absence of bone metastasis or even instances where bone metastasis is minimal. In these cases, it is evident that the tumor cells are generating a humoral factor which stimulates osteoclastic bone resorption, thus enhancing the entry of calcium into the extracellular fluid. This is recognized as the syndrome of humoral hypercalcemia of malignancy (HHM), marked by an elevation of parathyroid hormone-related protein (PTHrP). PTHrP is considered the primary factor responsible for this syndrome, though it cannot account for all its features. Current research is focused intensively on identifying and characterizing the humoral factors that enhance osteoclastic bone resorption.
PTHrp-related hypercalcemia
Most solid tumors associated with hypercalcemia secrete PTHrP. This protein has been extensively purified, cloned, and tested both in vitro and in vivo. It binds to and activates the PTH receptor and appears to have all the biological effects of PTH. PTHrP has also been found in various other cell types. However, despite its biological similarity to PTH, it cannot explain all the features of humoral hypercalcemia of malignancy. For example, features such as decreased bone formation, reduced production of active vitamin D, and metabolic alkalosis are found in HHM, but not in primary hyperparathyroidism.
The understanding is that those aspects of HHM that cannot be attributed to excess PTHrP may be due to other factors produced alongside PTHrP. It seems plausible that the complete HHM syndrome is caused by multiple mediators working together. For instance, transforming growth factor alpha (TGF-alpha), often produced by the same tumors that generate PTHrP, may be responsible for those elements of the hypercalcemic syndrome that cannot be linked to PTHrP. It is now clear that some of these factors, such as TGF-alpha, tumor necrosis factor (TNF), TGF, and IL-1, can modulate the response of bone cells and renal tubular cells to PTH and PTHrP.
Parathyroid hormone-related protein (PTHrP) was initially identified using in vitro tests for PTH in tumors originating from the lung, breast, and kidney. It is now recognized that PTHrP is expressed by many squamous cell carcinomas and has also been identified in T-cell lymphomas. It is a peptide composed of 141 amino acids and shares some similarity to parathyroid hormone in its first 13 amino acids. Its ability to bind to and activate the PTH receptor is likely why it can mimic the biological effects of PTH in bone, kidney, and gut.
TGF-α
TGF-α, working alongside PTHrP, is a strong inducer of osteoclast formation and the subsequent breakdown of bone. Its influence on bone absorption mainly targets osteoclast precursor cells. Ultimately, TGF-α triggers an increase in bone resorption and hypercalcemia.
PTH-mediated
Despite the evidence of PTH not being the main contributor to hypercalcemia related to a large number of nonparathyroid malignancies, it has been observed that most current immunoassays show reduced concentrations of immune-reactive parathyroid hormone. Furthermore, using a specific method to detect parathyroid hormone mRNA in most tumors related to hypercalcemia of malignancy, it was found that PTH mRNA was generally absent.
However, there have been some reported cases of nonparathyroid tumors that produce PTH. A case report of squamous cell carcinoma of the lung producing intact parathyroid hormone has been reported[2].
Clinical features of hypercalcemia
Signs and symptoms in patients with hypercalcemia due to malignancy often align with those observed due to other causes of hypercalcemia. The most prominent are neurological and gastrointestinal in nature. Neurological manifestations can range from fatigue and confusion to nightmares and stupor, escalating to a coma, or even death in severe cases. Gastrointestinal problems often include nausea, vomiting, and a lack of appetite. Constipation, while common in hypercalcemia due to primary hyperparathyroidism, is less frequent in patients with malignancies.
Other body systems, including the urinary tract, can also be affected by hypercalcemia. It invariably leads to a decrease in the ability to concentrate urine and resistance to the effects of antidiuretic hormone.
Kidney stones, common in hyperparathyroidism, are less common in hypercalcemia due to malignancy, likely due to the more transient nature of the condition.
Despite these similarities, hypercalcemia associated with malignancy has certain distinct characteristics. For example, it can develop quite quickly, leading to symptoms even at relatively lower serum calcium levels. Unlike those with chronic primary hyperparathyroidism, who may tolerate higher calcium levels without symptoms, individuals with malignancy can develop noticeable symptoms with comparatively modest increases in serum calcium if hypercalcemia sets in rapidly.
Another noteworthy aspect is that hypercalcemia symptoms, particularly those that affect the digestive system, can be easily misinterpreted in patients suffering from malignant diseases. For example, treatments like chemotherapy or radiation therapy often cause nausea and vomiting. Consequently, these symptoms, if caused by hypercalcemia, could be mistakenly attributed to the side effects of these treatments.
Treatment of Hypercalcemia of Malignancy
When to Begin Treatment
Patients with serum calcium levels greater than 13 mg/dl, or those with symptoms, should receive urgent treatment. On the other hand, asymptomatic patients with corrected total serum calcium below 12 mg/dl require close monitoring. Hypercalcemia linked to malignant diseases tends to progress rapidly, sometimes within just a few weeks. This differs from primary hyperparathyroidism, where hypercalcemia can remain stable for several years. This distinction guides the approach to treatment.
Selecting the Treatment
Treatment for hypercalcemia linked to malignancy can be divided into two types:
- Urgent treatment for patients with symptoms or having a serum calcium level greater than 13 mg/dl.
- Long-term treatment for patients experiencing mild hypercalcemia.
In numerous patients, especially those with myeloma, chemotherapy that effectively targets the malignancy can alleviate hypercalcemia. Furthermore, medications that could intensify or trigger hypercalcemia, such as thiazide diuretics, should be discontinued. If feasible, immobility (prolonged recumbency), which increases bone loss, should be avoided.
Urgent Management of Hypercalcemia
Rehydration
Administering intravenous saline (0.9% normal saline) is recommended as it leads to the excretion of sodium and calcium together. Care must be taken during its delivery, as it can cause high sodium levels, especially in patients who do not respond or lack a functioning thirst response. It is worth noting that hypernatremia, can occur due to loss of renal concentrating ability and insensitivity to antidiuretic hormone (nephrogenic diabetes insipidus) in the setting of hypercalcemia. This results in constant excretion of free water and an increase in serum sodium levels when large volumes of isotonic saline are administered, particularly to an unresponsive patient who does not experience normal thirst. Hypernatremia can be quickly reversed by administering fluids that are less concentrated than the body’s fluids.
Bisphosphonates and Calcitonin
For the urgent treatment of hypercalcemia, a combination of calcitonin and zoledronic acid is very effective. The maximum impact of zoledronic acid usually comes after 48 to 72 hours, while calcitonin begins to work within 12 hours. This combination works well because calcitonin quickly lowers serum calcium, although the effect does not last due to tachyphylaxis. Doses of 200 to 400 units of calcitonin can be administered intramuscularly or subcutaneously every 12 hours.
Glucocorticoid therapy
Also, glucocorticoids are generally safe and effective in acutely treating hypercalcemia, particularly if it is caused by a lymphoma.
Granulomatous conditions such as lymphoma are often associated with hypercalcemia due to certain physiological changes that occur within the body. These changes include an elevation in the activity of 1α-hydroxylase, a reduction in the breakdown of 1,25-dihydroxyvitamin D3 (1,25-OHD3), and the release of parathyroid hormone-related protein (PTHrP) and bone-resorbing cytokines like interleukin-6 (IL-6) from granulomatous lesions.
Within the macrophages present in granulomatous diseases, increased 1α-hydroxylase activity and reduced degradation of 1,25-OHD 3 are observed. These changes are driven by interferon-gamma (IFN-ϒ), a substance secreted from the granulomas themselves[3].
The primary treatment strategy for these conditions involves the use of glucocorticoids, such as prednisolone at doses ranging from 0.5 to 1 mg/kg/day, alongside specific therapy tailored to the underlying disease causing the granulomatous reaction. The glucocorticoids function by inhibiting the activity of 1α-hydroxylase and reducing the secretion of cytokines from the granulomatous lesions, which in turn helps manage the hypercalcemia.
Furosemide
Furosemide has been extensively used, but it is likely to be ineffective unless given in very high doses. When used in this way, it is inconvenient. Administration of furosemide in this manner requires the patient to be in an intensive care unit with careful monitoring of all fluids and electrolytes. Additionally, the patient must be fully rehydrated before initiating these doses of furosemide, as this diuretic can cause a decrease in the volume of fluid outside of the body’s cells, which could exacerbate hypercalcemia rather than alleviate it.
Small doses of furosemide have not been shown to have any beneficial effect on hypercalcemia, and could even worsen the condition if the patient is not fully hydrated. There is scant evidence that even larger doses of furosemide have any significant effects beyond that of the fluid load. Furosemide may be suitable for those rare patients with hypercalcemia who suffer from fluid overload, but in such cases, the treatment with furosemide is mainly for fluid overload, not because of its ability to lower serum calcium.
Denosumab
Denosumab, a monoclonal antibody, plays a significant role in the treatment of hypercalcemia of malignancy due to its ability to bind to RANK-L (receptor activator of Nuclear factor Kappa-B Ligand). This binding blocks the interaction between RANK-L (located in osteoblasts) and the RANK receptor found on osteoclasts.
The way denosumab works to prevent fractures is by suppressing osteoclast activity during the process of bone remodeling. With osteoclast activity in check, osteoblasts can effectively fill in the resorption voids left behind by these now inhibited osteoclasts, which in turn leads to an increase in bone mass[4].
Although denosumab is primarily categorized as an antiresorptive agent, facilitating remodeling-based bone formation, there is also evidence pointing to its role in promoting modeling-based bone formation. This dual action helps explain why there is an increase in bone mass at primarily cortical sites in patients treated with this powerful anti-resorptive agent.
In patients with impaired renal function (eGFR <30) in whom bisphosphonates are relatively contraindicated, denosumab serves as a reasonable anti-resorptive therapy.
Dialysis
Peritoneal or hemodialysis has occasionally been used to treat hypercalcemia. This treatment may temporarily lower serum calcium levels, but it does not address increased bone resorption, which is the main cause of hypercalcemia. Patients who undergo dialysis often have a high mortality rate, likely due to the severely ill state of patients who have severe hypercalcemia and kidney failure. Indeed, dialysis is recommended only for patients who have severe kidney failure combined with hypercalcemia. However, it should be understood that its beneficial effects will be temporary and additional forms of treatment will be required.
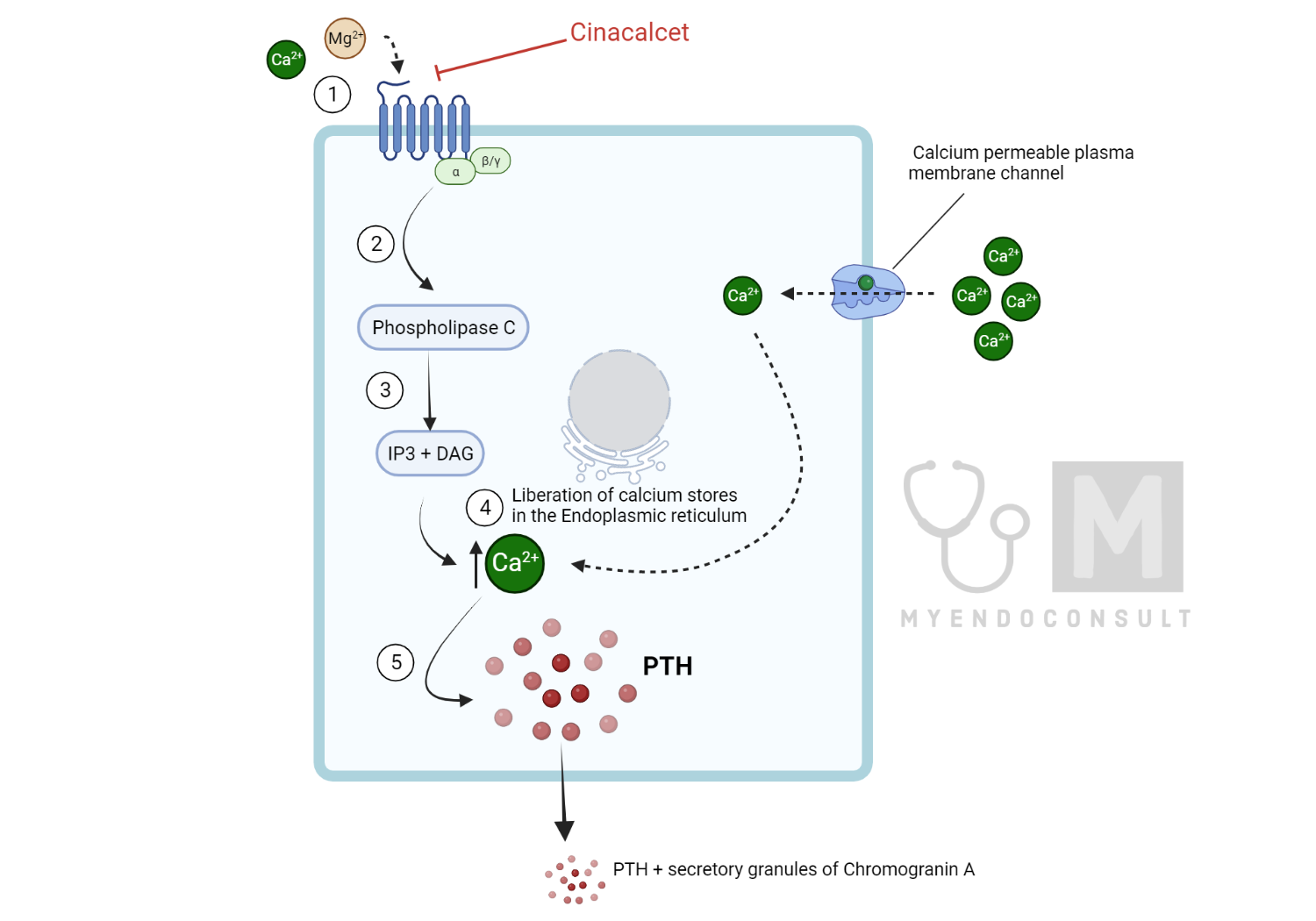
Calcimimetics
Calcimimetics function by improving the sensitivity of the Calcium-Sensing Receptor (CaSR) to serum calcium, thereby reducing the threshold required for receptor activation. This results in CaSR activation even when ionized calcium levels are relatively low, which consequently inhibits the release of parathyroid hormone (PTH). Unlike divalent cations such as calcium and magnesium that bind to the amino-terminal domain of the CaSR, calcimimetics attach to the receptor’s 7-transmembrane domain. This engagement triggers a structural change in the CaSR, leading to its increased response to extracellular calcium (a positive allosteric effect). Ultimately, calcimimetics decrease serum calcium by curbing PTH secretion and boosting renal calcium excretion. Cinacalcet serves as a prime example of a calcimimetic used in the medical treatment of primary hyperparathyroidism. Interestingly, there have been case reports of HHM being successfully treated with cinacalcet. The exact mechanism of action in non-PTH mediated hypercalcemia is, however, unclear at this time[5].
Long-term treatment of hypercalcemia of malignancy
Long-term treatment for hypercalcemia due to malignancy can involve glucocorticoids, bisphosphonates, and denosumab. The ideal chronic treatment would be a medication that can maintain normal calcium levels without side effects, especially during the final months of life. Many of these patients are in the advanced stages of the disease and are nearing the end of their life. The goal is to keep the patient as symptom-free and mobile as possible for as long as possible.
Bisphosphonates
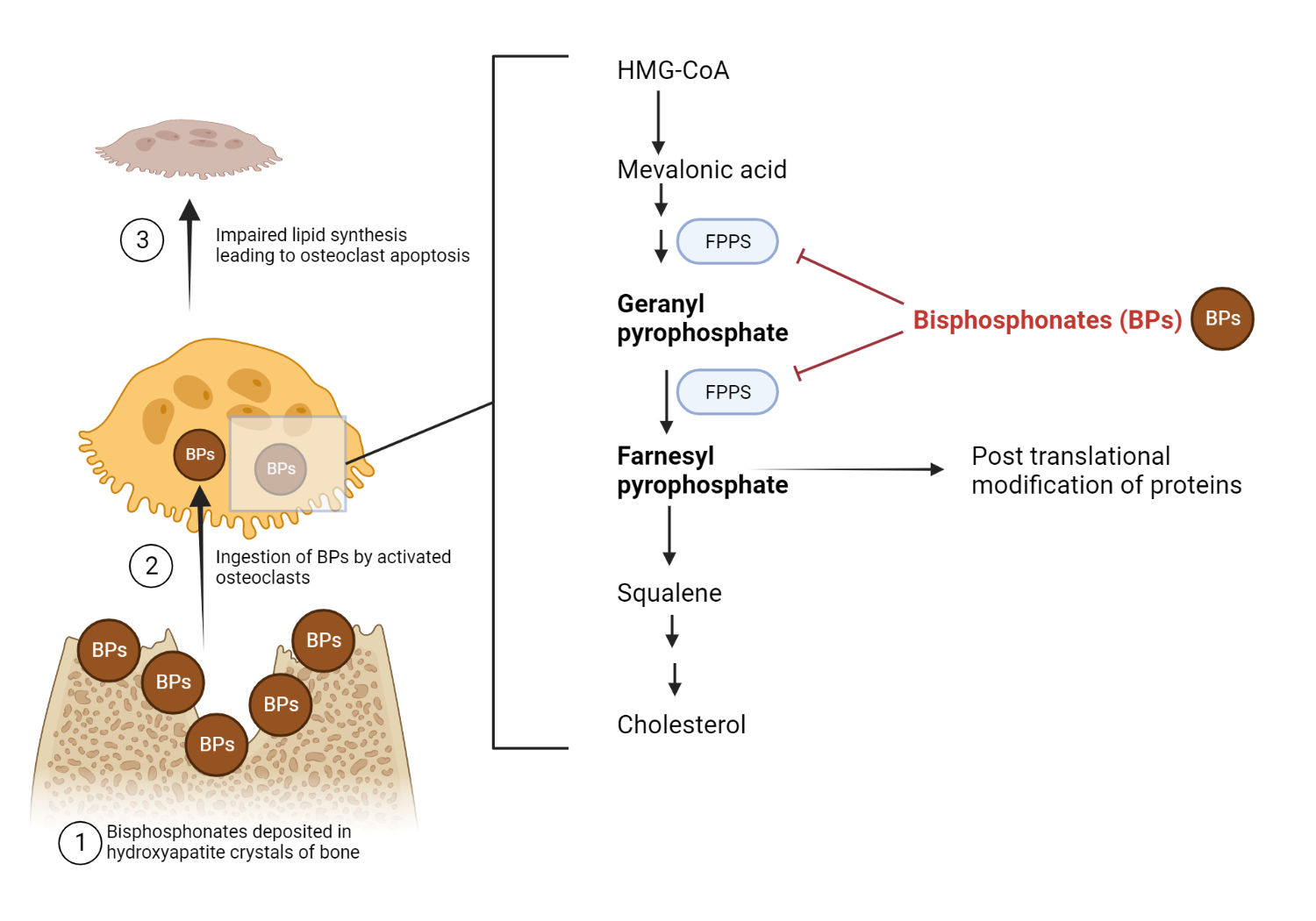
Bisphosphonates are a group of synthetic substances that are stable analogs of pyrophosphate. They differ from pyrophosphate in their basic structure, as the pyrophosphate P-O-P bond is replaced by a P-C-P bond in bisphosphonates. Therefore, bisphosphonates can resist enzymatic breakdown by pyrophosphatases found in the intestines and kidneys, resulting in a long half-life. They were initially developed to be added to toothpaste to prevent calcification and plaque buildup. This effect is attributed to their ability to disrupt crystal growth by attaching to hydroxyapatite, thereby blocking mineralization.
It was discovered during early studies on their action on bone that they also effectively inhibit osteoclastic bone resorption. This discovery underpins its utility in treating bone disorders.
Zoledronic acid is widely used in Europe and the United States and has been shown to be extremely effective when administered intravenously to patients with hypercalcemia of malignancy or osteolytic bone disease.
After a single intravenous administration of zoledronic acid, normal serum calcium levels should be achieved between 2 and 7 days, on average 3 days. The duration of action of zoledronic acid can vary between patients, but the serum calcium level often remains within the normal range for approximately 3-4 weeks.
Since it was discovered that new-generation bisphosphonates could alleviate bone pain and significantly reduce urinary calcium and hydroxyproline levels, indicative of decreased bone turnover, these drugs have been considered potential treatments for patients with painful bone lesions, even in the absence of hypercalcemia.
Glucocorticoids
Glucocorticoids demonstrate effectiveness in approximately 30% of all patients with hypercalcemia due to malignancy. They appear to be the most potent when used in patients with hematological malignancies. For those with solid tumors, glucocorticoids could decrease the amount of circulating Parathyroid Hormone-related Protein (PTHrP) in some individuals, likely as a result of influencing the transcription of the PTHrP gene.
These drugs prove to be highly beneficial for most patients who respond, especially considering the fact that many of such patients have a limited remaining life. If administered for a typically less than six-month period, the commonly known side effects of glucocorticoid therapy do not create significant concerns.
Denosumab
Denosumab, a monoclonal antibody, plays a significant role in managing hypercalcemia associated with malignancies. As was previously mentioned, its mechanism of action involves binding to the receptor activator of nuclear factor kappa-B ligand (RANKL) that’s found on osteoblasts. By doing this, it effectively blocks the activation of osteoclasts, which is usually triggered by the interaction between RANKL on osteoblasts and RANK on osteoclasts.
Preventing this interaction disrupts the process of bone resorption, a major cause of hypercalcemia in cancer patients, thereby helping to regulate calcium levels in the blood.
The typical dosing regimen for denosumab in this context is 120mg administered subcutaneously every 4 weeks. This treatment strategy offers a targeted approach to managing hypercalcemia in patients with malignancies, by directly interfering with the bone resorption process.
Frequently Asked Questions
How is PTH related peptide (PTHrp) mediated hypercalcemia different from primary hyperparathyroidism?
Hypercalcemia of malignancy and primary hyperparathyroidism are both conditions associated with elevated calcium levels in the blood, but they differ in their etiology, pathophysiology, and clinical presentation.
| Clinicopathological feature | Primary hyperparathyroidism | PTHrp-mediated hypercalcemia |
| Clinical course | Slowly progressive and intermittent hypercalcemia | Rapid onset of hypercalemia (in a few weeks) |
| Serum PTH | High or inappropriately normal | Low or undetectable |
| Serum Calcium | Mild to moderate hypercalcemia | Severe hypercalcemia |
| Serum phosphate | Low | Low |
| Serum Alkaline Phosphatase | Elevated | Normal |
| Bone formation markers (P1NP) | Stimulates | No effect |
| Bone resorption markers (CTX) | Stimulates | Stimulates |
References (Suggested)
1. Hypercalcemia and cancer: Differential diagnosis and treatment – Zagzag – 2018 – CA: A Cancer Journal for Clinicians – Wiley Online Library.
2. Nielsen, P.K., Rasmussen, A.K., Feldt-Rasmussen, U., Brandt, M., Christensen, L., and Olgaard, K. (1996) Ectopic production of intact parathyroid hormone by a squamous cell lung carcinoma in vivo and in vitro. J Clin Endocrinol Metab, 81 (10), 3793–3796.
3. Pufall, M.A. (2015) Glucocorticoids and Cancer. Adv Exp Med Biol, 872, 315–333.
4. Roukain, A., Alwan, H., Bongiovanni, M., Sykiotis, G.P., and Kopp, P.A. (2022) Denosumab for the Treatment of Hypercalcemia in a Patient With Parathyroid Carcinoma: A Case Report. Front Endocrinol (Lausanne), 12, 794988.
5. Asonitis, N., Kassi, E., Kokkinos, M., Giovanopoulos, I., Petychaki, F., and Gogas, H. (2017) Hypercalcemia of malignancy treated with cinacalcet. Endocrinol Diabetes Metab Case Rep, 2017, 17–0118.
Kindly Let Us Know If This Was helpful? Thank You!


