Hypothyroidism: Background
Hypothyroidism is a condition that affects approximately 5% of the global population. The most common cause of hypothyroidism in regions with sufficient iodine is Hashimoto’s disease, an autoimmune disease in which the body’s immune system attacks the thyroid gland.
The condition shows a pronounced female predominance, eight to nine times more common in women than men. Furthermore, the prevalence of hypothyroidism increases with age, reaching a peak between the ages of 30 and 50 years. In the United States, it affects approximately 4% of women aged between 8 and 24 years and approximately 21% of women over 74.
The Turning Point in Hypothyroidism Management
The landscape of hypothyroidism management changed significantly with the introduction of the thyroid-stimulating hormone (TSH) assay. This tool allowed clinicians to monitor treatment response, leading to the revelation that many patients were being overtreated and, in fact, were mildly thyrotoxic.
A key discovery was that peripheral tissues could convert thyroxine (T4) to triiodothyronine (T3). This led to the widespread use of levothyroxine (L-T4) monotherapy as the standard of care. Consequently, the use of desiccated thyroid extract gradually lost favor in clinical practice.
Despite this progress, around 5-10% of hypothyroid patients whose TSH is normalized with L-T4 monotherapy continue to experience persistent symptoms. These can range from cognitive impairment and depression to decreased psychomotor performance, which patients and clinicians often attribute to hypothyroidism.
Debating the Best Indicator of Euthyroidism in Hypothyroid Patients
A critical question arises when treating primary hypothyroidism with L-T4: Is TSH the best indicator of euthyroidism, or should T4 and T3 levels also be factored into management decisions?
There is variability in the mean, upper, and lower limits of TSH based on age, sex, and race. For example, TSH concentrations are generally higher in older individuals, women, and those of non-Hispanic descent. However, these age, sex, and race-specific cutoffs are not widely utilized for this assay, which could compromise its effectiveness as an indicator of euthyroidism.
On the contrary, the thyroid gland secretes T4 and T3 in a 14:1 ratio, and 20% of the circulating T3 comes directly from thyroid secretion. This might suggest that these two hormones should be given more weight in managing hypothyroidism.
The ongoing debates and research in this area reflect the complexity of hypothyroidism management and underscores the need for a personalized, patient-centered approach. As our understanding of the disease evolves, we hope to refine our diagnostic tools and treatment strategies to serve hypothyroid patients’ needs better.
The Role of Deiodinases and TSH in Thyroid Hormone Regulation
Deiodinases play a critical role in regulating thyroid hormone (TH). These enzymes convert the inactive form of thyroid hormone (T4) into its active form (T3) and help clear TH from the body.
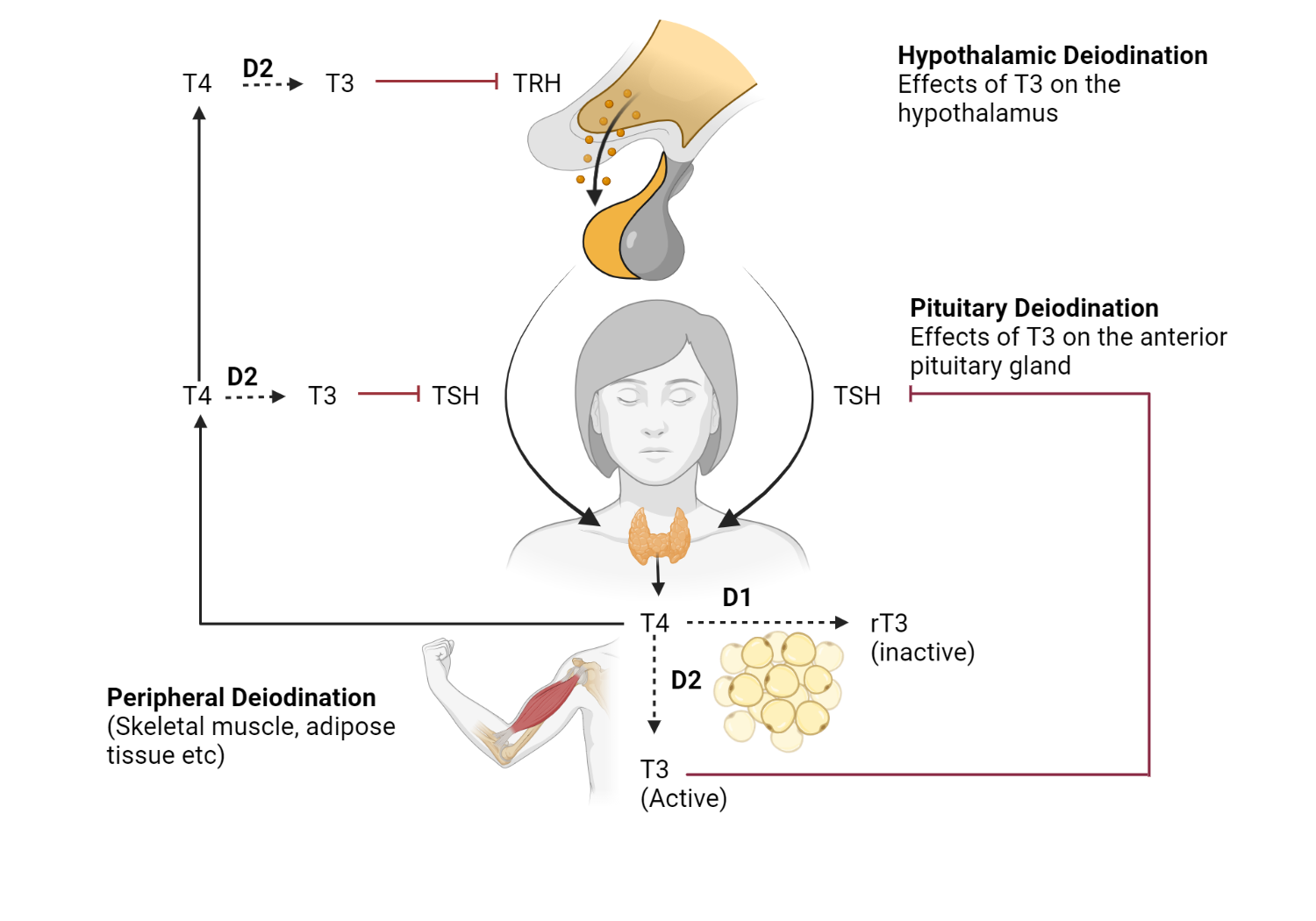
Type 1 deiodinase (D1) : D1 is responsible for approximately 20% of circulating T3 in the liver, kidney, and thyroid.
Type 2 Deiodinase (D2) : D2 is found in the hypothalamus, pituitary, central nervous system (CNS), brown adipose tissue (BAT), skin, heart, and skeletal muscle. It contributes to approximately 80% of circulating T3.
Type 3 deiodinase (D3) : Unlike D1 and D2, D3 is a “deactivating” deiodinase that participates in thyroid hormone clearance.
TSH Reflects Thyroid Hormone State in Hypothalamus/Pituitary
Relying on TSH as an indicator suggests euthyroidism at the hypothalamus-pituitary level but does not necessarily reflect the state of thyroid hormone at the tissue level. Observations of differing levels of thyroid hormone at the tissue level are primarily derived from rodent studies. These studies have shown that circulating T3 levels are not necessarily in equilibrium with tissue T3 levels.
In human studies, surrogate markers of thyroid hormone status in peripheral tissues, such as metabolic rate and cholesterol levels, have been used.
Can a better TH balance Be Achieved with a Combination of T3/T4 Replacement?
The effects of L-T4 administration on circulating T3 levels have been controversial in human studies. While some report normal levels, others report low levels.
Two extensive cross-sectional studies have shown lower T3 levels in L-T4-treated patients compared to control patients. In all the TSH quintiles analyzed, T4 was higher and T3 lower in patients treated with L-T4. Furthermore, 15% of these patients had T3 levels below the reference range, suggesting that L-T4 monotherapy may not fully normalize thyroid hormone status in all patients.
These findings have fueled ongoing discussions about the potential benefits of combination T3/T4 replacement therapy for hypothyroid patients. However, more research is needed to determine if such an approach could better balance thyroid hormones.
Recommendations for Clinical Practice
The sensitive nature of thyroid stimulating hormone (TSH) to circulating thyroxine (T4) makes it an effective biochemical marker for diagnosing hypothyroidism. Despite ongoing discussions about the potential benefits of incorporating T3 and T4 levels into the management of hypothyroidism, current studies do not support a change in practice from reliance on TSH in diagnosing and treating primary hypothyroidism.
Most people with hypothyroidism respond biochemically and symptomatically well to levothyroxine (L-T4) monotherapy when TSH is used as the biomarker to guide dose adjustments.
What is the phenomenon of persistent hypothyroidism?
Although most people on L-T4 therapy fare well, a subset of patients reports perceived poor health status. Compared to controls of the same age and sex, L-T4 patients on L-T4 have exhibited reduced psychological well-being. This phenomenon was formally documented for the first time in 2002.
Certain factors can potentially complicate the assessment of persistence in patients on L-T4 therapy. These include:
- The presence of multiple chronic diseases that impact the quality of life
- Polypharmacy
- The presence of underlying conditions that affect symptomatology
Furthermore, there is a considerable overuse of L-T4 without a precise biochemical diagnosis of hypothyroidism. About a third of patients prescribed L-T4 show no biochemical evidence of thyroid dysfunction. Thus, it is vital to consider whether persistent symptoms might be related to lower peripheral T3 levels after ruling out all other causes.
Deiodinase Polymorphisms: An Unresolved Piece of the Puzzle?
Polymorphisms involving deiodinases occur in a small percentage of the population and may explain why a small group of patients still have symptoms despite L-T4 therapy. Two examples are D2‐Thr92Ala and D2‐ORFa‐Gly3Asp, which are associated with lower enzyme activity. However, current evidence does not support a relationship between D2 polymorphism, thyroid parameters, and adverse clinical consequences. As a result, the implications of these polymorphisms in managing hypothyroidism are unclear.
Other Potential Confounders of Persistent Hypothyroidism
Traditionally, thyroid hormone action occurs by binding to nuclear receptors and affecting the transcription of tissue-specific genes. However, non-canonical pathways for thyroid hormone action exist and require further research. These could explain the presence or absence of symptoms in patients undergoing replacement therapy.
In conclusion, while TSH remains the cornerstone of diagnosing and managing hypothyroidism, the persistence of symptoms in a subset of patients underscores the complex nature of this condition and the need for continued research. This will help optimize treatment strategies to accommodate individual patient needs.
Addressing Hypothyroidism: Considering the T3 / T4 Combination Therapy
Although levothyroxine monotherapy (L-T4) remains the standard treatment for hypothyroidism, some research has focused on the potential benefits of a combination of triiodothyronine (T3) and T4. However, most clinical trials conducted to date do not support the superiority of combination T3/T4 therapy over L-T4 monotherapy. Furthermore, most studies do not indicate any difference in patient preference between the two treatment approaches.
These trials varied in terms of sample size, achieved TSH and T3 levels, administered L-T4:L-T3 ratios (which often differed significantly from the physiological 14: 1 ratio), and duration of study periods.
Recommendation for Clinical Practice
Observational studies and surveys suggest that patients with hypothyroidism treated with L-T4 report declines in various quality of life (QOL) parameters and psychological well-being compared to euthyroid controls. However, concrete evidence supports the improvement with the addition of L-T3 to the treatment regimen.
Multiple factors are likely to impact these assessments and perceptions of well-being. If TSH normalizes and symptoms persist, evaluation of other causes should be sought. In such cases, checking L-T3 levels can be considered, and L-T3 can be introduced as an addition to L-T4 treatment. It is important to note that there are minimal risks associated with L-T3 use, provided it is closely monitored.
Logistical Concerns Regarding L-T3
A key logistical concern surrounding the use of L-T3 is the lack of long-term studies understanding its impact on clinically significant outcomes. Additionally, the administration of T3 often requires multiple daily doses to maintain sustained levels. Sustained release T3 presents an alternative, resulting in a smaller peak in T3 levels, followed by a more sustained plateau relative to L-T3. The effects on TSH are similar to those seen with L-T3.
In conclusion, while L-T4 monotherapy remains the standard approach to the treatment of hypothyroidism, the potential benefits of a combination T3/T4 therapy cannot be dismissed. Continued research is necessary to explore this combination strategy’s clinical efficacy and safety, which could improve the therapeutic landscape for people with hypothyroidism.
Recommendations for future studies that evaluate the role of T3/T4 Therapy
The management of hypothyroidism, a condition marked by insufficient thyroid hormone production, has been a topic of ongoing debate. The primary treatment has long been levothyroxine (L-T4) therapy. However, a minority of patients remain symptomatic despite their use, sparking interest in combination triiodothyronine (T3) / L-T4 therapy. This article proposes recommendations for future research to assess the role of T3/T4 therapy more effectively.
Patient Selection Criteria
To evaluate the impact of T3/T4 therapy, it is crucial to target patients dissatisfied with their current L-T4 treatment and who continue to show symptoms despite such treatment. The inclusion of patients with little residual thyroid tissue can minimize the confounding of endogenous thyroid hormone production.
Quality of Life Assessment
The use of thyroid-specific Quality of life (QOL) questionnaires should be standardized to accurately assess patients’ experiences. More representative results can be achieved by including patients with comorbidities such as diabetes mellitus, cardiovascular disease, hypertension, and treated depression.
Standardized Blood Sampling and Assay Methodology
The timing of blood sampling for T3 should be consistent across studies. Furthermore, the T3 assay used should be uniform, with evidence suggesting that Total T3 (TT3) assays outperform Free T3 (FT3) assays. It is also crucial to consider the timing of these measurements due to the variability in the magnitude and timing of peak hormone levels. Measurement of trough levels can provide a more reliable assessment.
Administration and Dosage of Drugs
Clinical trials should use at least a twice-daily dosing regimen rather than once daily, ideally using a sustained-release formulation of T3. Combination therapy should strive to mimic a 14:1 ratio and achieve a physiological level of thyroid-stimulating hormone (TSH).
Power, Duration, and Design of the Study
Studies need to have the appropriate power to detect significant differences in quality of life. Longer studies, ideally of 9 to 12 months, are necessary, although this may pose recruitment challenges. The study design should aim to show superiority, although noninferiority can establish safety relative to standard care.
Factoring in the Etiology of Hypothyroidism
The cause of hypothyroidism should be factored into the study design, as it may influence the response to therapy. Additionally, it is recommended to avoid combination therapy in patients who are pregnant, trying to conceive, or have arrhythmias or established cardiovascular diseases.
Conclusions
Despite L-T4 remaining the mainstay of hypothyroidism treatment and TSH being the most valuable biomarker for diagnosis, persistent symptoms in a small percentage of patients require a closer look at the potential role of T3/T4 combination therapy. More studies are needed to understand the multifactorial nature of these symptoms.
Take-Home Point
Combination therapy should be individualized, and current clinical data do not support a change in practice guidelines from the use of L-T4 monotherapy as the standard treatment approach for hypothyroidism. Further research following these outlined recommendations may provide additional information on the potential benefits and drawbacks of T3/T4 therapy.
Kindly Let Us Know If This Was helpful? Thank You!


