The mechanism of action of pegvisomant and its clinical application in the management of acromegaly will be reviewed.
Physiology
Growth hormone and IGF-1 pathway : The growth hormone receptor (GH-R) belongs to the cytokine receptor family(1) and is composed of extracellular (binds its cognate ligand, i.e., GH) transmembrane and cytosolic domains(2). See figure 1.0
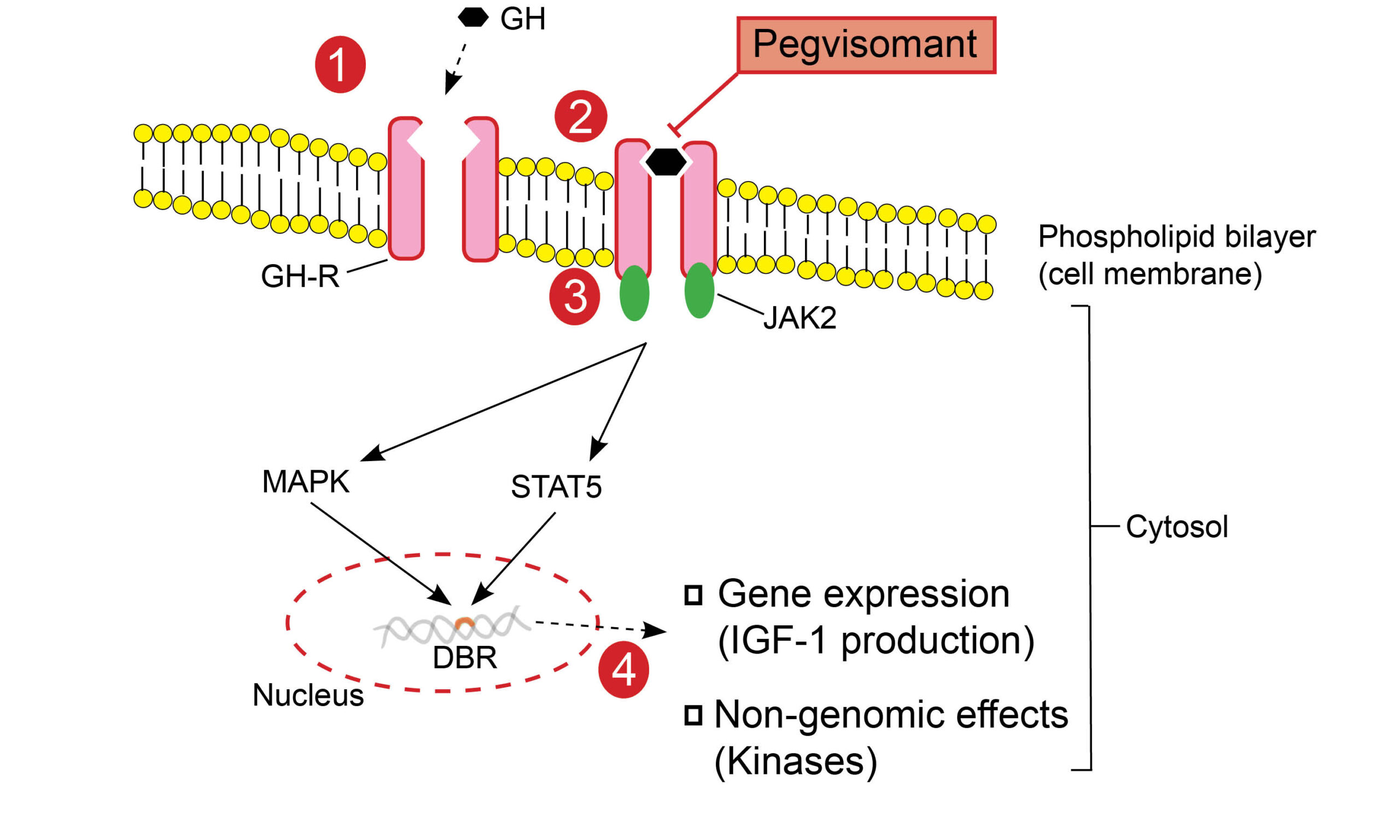
Fig. 1.0 Growth hormone receptor and the intracellular effects of GH to GHR binding. GH binds to the GHR, leading to dimerization and consequent structural change in the receptor. This is a critical step that initiates the Janus-Kinase (JAK)-signal transducer and activator of transcription (STAT) intracellular signaling pathway. Phosphorylation of tyrosine residues in both the GHR transmembrane domain and STAT molecules is mediated by activated JAK2 tyrosine kinase(3). There are various STAT molecules involved in specific intracellular. STAT5 (transcription factor), for example, is translocated from the cytoplasm to the nucleus and binds to DNA at specific DNA binding regions(DBRs)(4), which encode protein sequences(5). Mitogen-activated protein kinase (MAPK) activation by JAK2 ultimately exerts genomic (gene expression) and non-genomic effects. GH mediates the production of IGF-1 in various tissues apart from the liver, including growth plate chondrocytes, adipose tissue, and skeletal muscle(5).
Dehkhoda F et al. (2018) The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol 9:35
Table 1.0 Gene sequences encoding IGF-1 production in specific organs
| Organ | Gene sequence(s) |
| Liver | Socs2 |
| Skeletal muscle | Igf1 |
| Fat (Adipose tissue) | Fos, Jun, and Igf1 |
| Bone (Chondrocyte) | Igf1 |
Socs2 suppressor of cytokine signaling 2, Igf1 insulin-like growth factor 1, Jun proto-oncogene, Fos proto-oncogene
Adapted from Chia DJ (2014) Minireview: Mechanisms of Growth Hormone-Mediated Gene Regulation. Mol Endocrinol 28:1012–1025
Mechanism of action
Pegvisomant has structural homology with endogenous GH apart from the substitution of nine amino acids. Pegylation (the process of attaching polyethylene glycol to protein) of GH changes its pharmacokinetic properties and making it hypoallergenic(6).
Pegvisomant, due to its similarity to GH, can occupy the GH receptor pocket, depriving the receptor of direct activation by GH. More importantly, it does not activate the GHR (antagonistic action) because it induces defective dimerization of the receptor, thus preventing subsequent signal transduction pathways (JAK-STAT signaling) and eventual IGF-1 production(7).
Practice Guide
- Pegvisomant can cause an increase in the size of GH secreting tumors; it is thus recommended to monitor tumor size with serial pituitary MRIs(8). Co-administration of somatostatin analogs and pegvisomant can reduce the risk of tumor re-expansion compared to pegvisomant monotherapy(9).
- Pegvisomant, being a growth hormone receptor antagonist, causes an increase in GH levels, thus limiting the utility of GH assessments during monotherapy with this agent. As a result, assessment of treatment response requires serial monitoring of serum IGF-1 levels(8).
- Hepatic enzyme elevation occurs during Treatment and resolves after cessation of therapy. Liver function tests (LFTs) should therefore be routinely monitored during treatment. As a consequence, patients with unexplained elevations in LFTs should not start pegvisomant(10).
Clinical Trial Evidence
The pivotal study that led to the approval of pegvisomant was published in 2000. It was a phase III study comparing various doses of pegvisomant with placebo(7). Additional long-term safety data are available from the ACROSTUDY database. The ACROSTUDY registry is a multinational, prospective, Phase IV (post-marketing surveillance) study of patients with acromegaly treated with pegvisomant(11,12)
Key Message
There is a low but clinically significant risk of tumor reexpansion for patients on pegvisomant monotherapy, although this should not preclude its use (ACROSTUDY).
A randomized, double-blind, placebo-controlled study comparing various doses of pegvisomant with placebo in patients with acromegaly over a 12-week study period. A total of 112 subjects with confirmed acromegaly, status-post pituitary surgery, radiation therapy, drug therapy, or treatment-naïve. The study participants were randomized to various doses of daily administered subcutaneous pegvisomant (10mg,15mg, or 20mg) or comparable placebo. The primary outcome was defined as a mean change in IGF-1 compared to baseline. There was a mean decrease in IGF-1 of 4%, 26.7%, 50.1% and 62.5% in the placebo, 10mg,15mg and 20mg doses, respectively. Various doses of pegvisomant compared to placebo resulted in a clinically significant decline in IGF-1 and improvement in clinical features of acromegaly(7).
References
- Waters MJ, Shang CA, Behncken SN, Tam SP, Li H, Shen B, et al. Growth hormone as a cytokine. Clin Exp Pharmacol Physiol. 1999 Oct;26(10):760–4.
- Brooks AJ, Wooh JW, Tunny KA, Waters MJ. Growth hormone receptor; mechanism of action. Int J Biochem Cell Biol. 2008 Jan 1;40(10):1984–9.
- Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J. 2015 Feb 15;466(1):1–11.
- Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol. 2018 Feb 13;9:35.
- Chia DJ. Minireview: Mechanisms of Growth Hormone-Mediated Gene Regulation. Mol Endocrinol. 2014 Jul 1;28(7):1012–25.
- Veldhuis JD, Bidlingmaier M, Bailey J, Erickson D, Sandroni P. A Pegylated Growth Hormone Receptor Antagonist, Pegvisomant, Does Not Enter the Brain in Humans. J Clin Endocrinol Metab. 2010 Aug;95(8):3844–7.
- Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000 Apr 20;342(16):1171–7.
- Tritos NA, Biller BMK. Pegvisomant: a growth hormone receptor antagonist used in the treatment of acromegaly. Pituitary. 2017 Feb;20(1):129–35.
- Giustina A, Arnaldi G, Bogazzi F, Cannavò S, Colao A, De Marinis L, et al. Pegvisomant in acromegaly: an update. J Endocrinol Invest. 2017;40(6):577–89.
- Hodish I, Barkan A. Long-term effects of pegvisomant in patients with acromegaly. Nat Clin Pract Endocrinol Metab. 2008 Jun;4(6):324–32.
- Grottoli S, Maffei P, Bogazzi F, Cannavò S, Colao A, Ghigo E, et al. ACROSTUDY: the Italian experience. Endocrine. 2015 Feb;48(1):334–41.
- Tritos NA, Mattsson AF, Vila G, Biller BMK, Klibanski A, Valluri S, et al. All-cause mortality in patients with acromegaly treated with pegvisomant: an ACROSTUDY analysis. Eur J Endocrinol [Internet]. 2020;182(3). Available from: https://eje.bioscientifica.com/view/journals/eje/182/3/EJE-19-0794.xml
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
Kindly Let Us Know If This Was helpful? Thank You!


