Pharmacological doses of iodide were first noted by Plummer (1923) to improve symptoms of thyrotoxicosis and to reduce the risk of subtotal thyroidectomy, at that time the only available treatment for Graves’ disease.
Rationale for radioiodine in hyperthyroidism
Radioiodine may be administered either per os or intravenously. In Graves’ disease, 30-90 % of the radioactivity will rapidly be taken up by the hyperfunctioning thyroid gland, while the remainder will be excreted in the urine. The radioiodine is slowly lost from the gland into the blood as a labeled hormone. The most frequently used iodine isotope, I-131, has a physical half-life of 8 days. Its decay produces both β-particles with a mean tissue pathway of about 1 mm and γ-quanta.
The β-particles are responsible for 90 % of the therapeutic effect; on being absorbed, they destroy thyroid tissue without affecting neighboring organs, blood vessels, nerves, or connective tissue. The γ-ray emission of I-131 is used mainly for diagnostic purposes.
The chemical amount of iodine introduced into the body by radioiodine treatment is so small that it has no biochemical or pharmacological effect (1 mCi of carrier-free 131 I = 0.01 mcg iodine).
Precautions with radioactive iodine
Indications for radioiodine vary from one center to the other. In some clinics, radioiodine is used whenever it is not contraindicated, and 80 % or more of patients with Graves’ disease are treated in this way. As far as risks (except hypothyroidism), costs, and inconvenience to the patient are concerned, radioiodine is indeed unsurpassed by any other treatment for Graves’ disease.
There are, however, several contraindications to the use of radioiodine. Since the fetal thyroid accumulates iodine from the third month of gestation onwards, radioiodine should never be given during pregnancy.
Furthermore, radioiodine is excreted into the milk and should, therefore, not be given to a nursing mother. Also, the thyroid gland appears to be very sensitive to the carcinogenic effect of radiation in childhood.
Relative contraindications are large diffuse and large nodular goiters with rapid radioiodine turnover since unduly high doses of radioactivity have to be applied, producing whole-body irradiation above permissible limits. Ophthalmopathy (Thyroid Eye Disease) is a relative contraindication to radioactive iodine treatment due to the risk of radioiodine-induced exacerbation of TED.
Strong indications for radioiodine are:
- Recurrent hyperthyroidism after subtotal thyroidectomy. In these cases, radioiodine is the treatment of choice.
- Recurrent hyperthyroidism after drug treatment (methimazole or propylthiouracil). In these cases, the options are radioiodine or surgery;
- Established Graves’ disease in patients over age 25, whether with diffuse or nodular goiter.
There are several potential risks in radioiodine therapy:
- Carcinogenic effect on the thyroid tissue;
- Production of leukemia due to bone marrow irradiation;
- Production of recessive mutations due to gonadal irradiation;
- Hypothyroidism.
- Severe thyroid eye disease
- Severe radiation-induced thyroiditis (severe neck pain, fever, and hyperthyroidism)
All these risks have to be weighed against those of surgery or drug treatment. Although isolated cases of thyroid neoplasia after radioiodine treatment have occurred, it is generally agreed that the incidence is extremely low and probably no higher than in a control population.
Chromosomal changes have been found in the lymphocytes of radioiodine-treated patients the incidence of leukemia was no higher than in the general population.
The radiation dose received by the gonads in the course of radioiodine treatment (about 20-30 rad) is in the same order of magnitude as the dose received during a gastrointestinal radiologic examination.
The genetic risk to the population induced by radioiodine is only a tiny fraction of that produced by radiation from natural sources, and the expected rate of mutations in the descendants of radioiodine-treated parents is only minimally higher than in untreated parents.
The exact mode of action of 131I has not yet been established, but it is generally assumed that it damages the thyroid cell nucleus and thereby interferes with cell division. Since there is probably a slow turnover of cells, inhibition of cell replication will progressively diminish the number of active cells.
The high risk of hypothyroidism after radioiodine places a great responsibility upon the doctor. First, he has to inform the patient of this risk and tell him about the most common symptoms of hypothyroidism.
Second, the patient must be followed up for the rest of his life and examined by the doctor at least once each year. Failure of the patient to keep his appointment should make the doctor suspect that he has become hypothyroid, and every effort should be made to contact him and to bring him under medical supervision. It is the fact that hypothyroidism is latent for some years, develops insidiously, and may easily be mistaken for cerebral arteriosclerosis in older persons that makes this lifelong followup mandatory in all patients treated with radioactive iodine.
Estimation of the dose of radioactive iodine
Numerous methods are in use for the calculation of the dose of 131I. All the methods use at least one variable, the estimated thyroid weight, in the computation. Thus, in most centers in the United States, the gland weight is estimated by palpation, and 80-160 microCi of 131I per g weight of thyroid tissue is given.
The calculations used include several other variables in an attempt at more exact control of the radiation energy delivered to the tissue. The following unit is in use as a measure of radiation energy: rad (radiation absorbed dose): 1 rad corresponds to 100 erg of energy delivered to 1 gram of tissue.
The following 5 factors or variables are considered in calculations of dosage for individual cases:
- Physical properties of 131I: The β-emission energy, which is the one relevant to its therapeutic effect, is 0.205 MeV, including conversion electrons. With additional components of the isotopic decay, in particular γ-emission, the total energy is 0.248 MeV. One mCi of 131I delivers 0.56 rad per hour in 1 g of tissue, provided the isotope distribution is homogeneous.
- Volume or weight of the thyroid gland, which may be estimated by palpation or by planimetry of thyroid scintigrams. The following formula: Weight = 0.323 x average height of lobes x surface of gland in a dorsoventral scintiscan.
- Percentage or fraction of the 131I dose taken up by the thyroid. Several measurements must be taken after a diagnostic dose to obtain the peak uptake.
- Distribution of 131I within the thyroid tissue. Since radioiodine is unevenly distributed in thyroid tissue the resultant intrathyroidal dose lattice is nonuniform. The maximum dose at certain sites can be 3.4 to 25 times greater (mean value of 10 times greater) than the calculated average dose. The estimated minimal dose may be quite important, since it may allow the survival of normal thyroid tissue, which will maintain euthyroidism.
Furthermore gross inhomogeneity of radioiodine distribution such as occurs in nodular goiters usually calls for an increase in the dose of 131I.
- Rate of release of radioiodine from the thyroid gland. This is included in the final’ formula as effective half-life (Teff), which is·obtained from the equation:
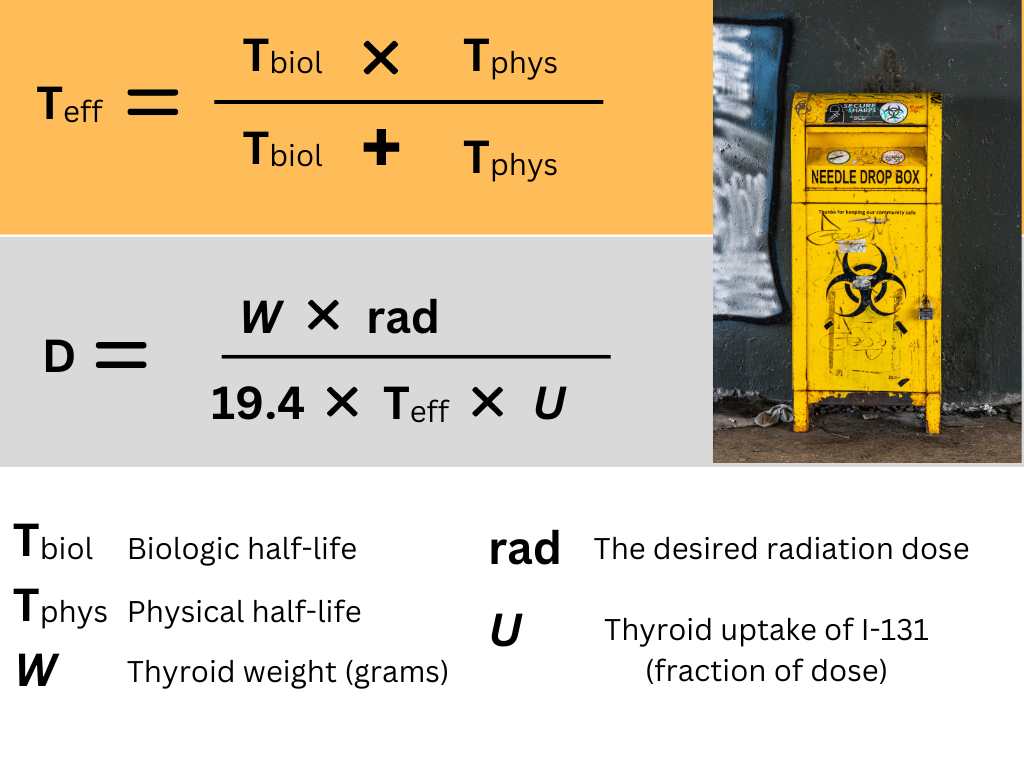
Table 1. Guidelines for determining the radioiodine dosage (rad) in Graves’ disease
| I | II | III | |
| Very small goiter | 3000 | 5000 | 7000 |
| Goiter <120grams | 4000 | 7000 | 10,000 |
| Goiter >120grams | 5000 | 9000 | 12,000 |
Grade I in patients with risk factors for thyroid eye disease (TED) in whom high doses might aggravate eye disease. Grade III in elderly patients with heart failure, in whom rapid control of thyrotoxicosis is desirable
Indications and contraindications for various forms of treatment of thyrotoxicosis
Radioiodine
a) Never during pregnancy;
b) Never under age 25, except in a few special cases;
c) Treatment of choice for relapse after operation.
Subtotal thyroidectomy
a) Always in rapidly growing goiters with suspected malignancy;
b) Often in large nodular goiters with pressure symptoms (radioiodine may be an alternative).
Thionamide drugs
a) Always preoperatively before thyroidectomy.
b) In all cases of Graves’ disease during childhood;
c) Always in thyrotoxic crisis.
d) In most cases during pregnancy (thyroidectomy is an alternative).
e) Sometimes as an adjunct after radioiodine to hasten appearance of euthyroidism;
f) In selected adult patients as a 1-year course;
g) Not recommended in cases of relapse after a first course of drugs or after surgery.
The results of radioiodine treatment are usually quite satisfactory if the high risk of hypothyroidism is disregarded.
Thyrotoxicosis is controlled within 3 to 6 months in most cases. After this time a second dose can be given if needed. The slow onset of action is a disadvantage, but control is obtained more quickly by beginning administration of a thionamide drug 10-14 days after the 131-I dose.
Quite a number of patients are pretreated with thionamide drugs before the decision to use 131 I is made; this is then usually given two to three weeks after the drug has been stopped.
It is worth noting that pretreatment with methimazole or propylthiouracil lowered the cure rate of 131I, while carbimazole (mainly used in Europe) had no such effect.
Some patients show a transitory exacerbation of thyrotoxic symptoms (due to radiation thyroiditis); however, in our experience, however, this is a very rare complication. A few patients complain of pain in the neck region between the second and tenth day after 131I.
Kindly Let Us Know If This Was helpful? Thank You!


