Physiology
Stages of Insulin Release Following a Meal
Insulin is instrumental in controlling high blood glucose levels. It encourages glucose absorption into cells and represses the liver’s glucose production. The process of insulin release is multifaceted, taking place in two distinct stages, known as the first and second phases of insulin release.
First Phase of Insulin Release: This phase begins within a few minutes of eating a meal, displaying a swift yet short-lived response (around 10 minutes), which leads to a rise in arterial glucose concentration. This stage involves the swift release of pre-stored insulin granules. It is believed to be triggered by the stimulation of the pancreas’s beta cells by glucose and other nutrients from the meal. The first phase of insulin release serves to promptly reduce blood glucose levels by promoting glucose absorption into cells and inhibiting hepatic glucose production.
Second Phase of Insulin Release: This phase occurs several minutes to hours after the first phase, offering a slower, extended response to high blood glucose levels. It is thought to be controlled by the release of additional hormones, such as incretins, that are produced in response to a meal. The second phase of insulin release aids in maintaining steady blood glucose levels for several hours after a meal, by encouraging glucose uptake into cells and inhibiting the liver’s glucose production.
A variety of circulating components, including glucose, incretins, amino acids, ketone bodies, lactic acid, and fatty acids, promote the release of insulin by pancreatic beta cells. Moreover, glucose-induced insulin release plays a significant role in deciding the extracellular insulin concentration in both fasting and post-meal states. Glucose enters pancreatic beta cells through the active glucose transporter 2 (GLUT-2). Glucokinase then prompts the metabolism of glucose into glucose-6-phosphate, which leads to the production of intracellular ATP through glycolysis. NADH and FADH2 created from glycolysis are further integrated into the mitochondrial electron transport chain to increase intracellular ATP levels. As a result, the high ATP to ADP ratio in the pancreatic beta cell inhibits the ATP-sensitive potassium channels in the plasma membrane. This leads to the depolarization of the plasma membrane, the opening of voltage-gated calcium channels, and the influx of calcium via the L-type voltage-gated calcium channels. An increase in intracellular calcium then aids in merging the insulin secretory granules with the pancreatic plasma membrane. Finally, insulin, c peptide, proinsulin, and other secretory products are released through a process of exocytosis.
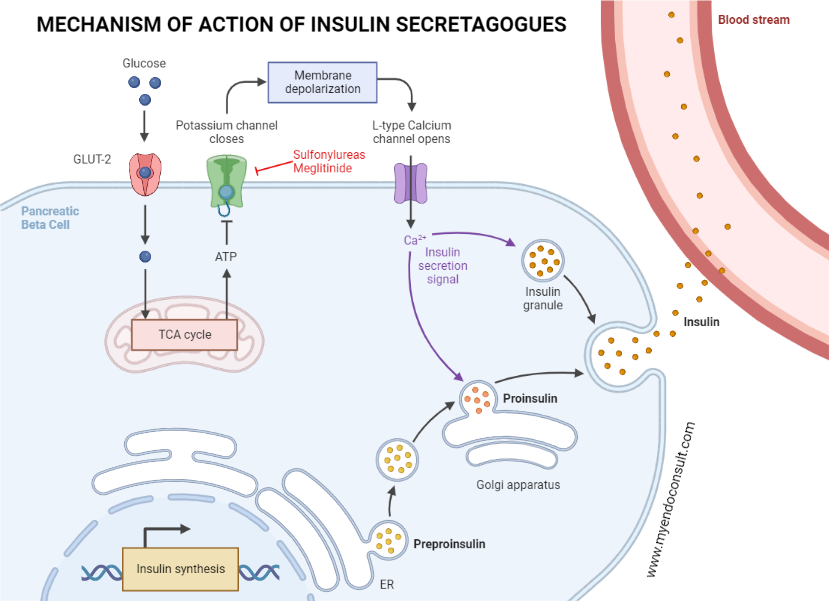
The transport of glucose into the pancreatic beta-cell is facilitated by glucose transporter 2 channels. This is followed by the conversion of glucose into glucose-6-phosphate, an action mediated by glucokinase. ATP, produced as a result of glycolysis, impedes the function of potassium channels on pancreatic cell membranes, leading to the depolarization of the cell membrane. Once the pancreatic beta-cell membrane is depolarized, L-type voltage-gated calcium channels within the pancreas are activated, allowing calcium to enter the pancreatic beta-cell. An increase in intracellular calcium levels then triggers the release of insulin reserves into the systemic circulation.
Fatty acids serve as an alternative trigger for insulin release. In pancreatic beta cells, fatty acids undergo beta oxidation and oxidative phosphorylation. The ensuing production of ATP inhibits the ATP-dependent or sensitive potassium channels, leading to the eventual release of insulin through exocytosis, a process similar to glucose-induced insulin release. Furthermore, fatty acids in the gut stimulate the secretion of incretins, which then enhance the release of insulin by pancreatic beta cells.
Mechanism of Action of Repaglinide (meglitinide)
Meglitinides enhance early-phase insulin release by directly obstructing the ATP-dependent potassium channels in the pancreatic beta cell. This action takes place irrespective of the intracellular ATP to ADP ratio. The blockade of the potassium channels leads to a shift in the electrical balance of the cell membrane, known as depolarization. Following this, voltage-gated calcium channels open, allowing a flood of calcium into the cell. Ultimately, this culminates in the exocytosis, or expulsion, of insulin-loaded granules from the cell.
Practice Guide for Repaglinide
- Repaglinide (Prandin) is taken orally in doses ranging from 0.5 to 4mg, two to three times daily, with a maximum daily dose of 16mg. It’s best consumed about thirty minutes before a planned meal. If a meal is skipped, the corresponding repaglinide dose should also be skipped to prevent low blood sugar, or hypoglycemia. The drug takes effect roughly thirty minutes after ingestion, and its action lasts between 4 to 6 hours. A key benefit of repaglinide is its rapid start and end of action, making it effective in managing spikes in blood sugar levels after meals.
- As an insulin stimulant, repaglinide can lead to weight gain, much like sulfonylureas. Other potential side effects include sinus inflammation, diarrhea, and joint pain.
- Repaglinide is mainly cleared from the body via the bile, making it a good option for patients with chronic kidney disease who may not be able to tolerate metformin or sulfonylureas.
- Repaglinide is not recommended for patients who are currently taking clopidogrel or gemfibrozil.
References
- Pakkir Maideen NM, Manavalan G, Balasubramanian K (2018) Drug interactions of meglitinide antidiabetics involving CYP enzymes and OATP1B1 transporter. Ther Adv Endocrinol Metab 9:259–268
- Derosa G, Mugellini A, Ciccarelli L, Crescenzi G, Fogari R (2003) Comparison between repaglinide and glimepiride in patients with type 2 diabetes mellitus: a one-year, randomized, double-blind assessment of metabolic parameters and cardiovascular risk factors. Clin Ther 25:472–484
- Derosa G, Mugellini A, Ciccarelli L, Crescenzi G, Fogari R (2003) Comparison of glycaemic control and cardiovascular risk profile in patients with type 2 diabetes during treatment with either repaglinide or metformin. Diabetes Res Clin Pract 60:161–169
- Jovanovic L, Hassman DR, Gooch B, Jain R, Greco S, Khutoryansky N, Hale PM (2004) Treatment of type 2 diabetes with a combination regimen of repaglinide plus pioglitazone. Diabetes Res Clin Pract 63:127–134
- Bennett K, James C, Hussain K (2010) Pancreatic β-cell KATP channels: Hypoglycaemia and hyperglycaemia. Rev Endocr Metab Disord 11:157–163
Kindly Let Us Know If This Was helpful? Thank You!


