Insulin plays an important role in various metabolic processes throughout our body. It is particularly crucial for controlling blood glucose levels, ensuring it is either stored for future use or utilized immediately.
Role of Insulin in Glucose Control
Insulin facilitates the transfer of glucose from the bloodstream into different types of tissues, such as skeletal and cardiac muscle as well as fat tissues. Most body tissues are equipped with insulin-independent glucose transporters (GLUT proteins) which allow a small amount of glucose to enter the cells. However, in skeletal and cardiac muscle cells and fat cells, a significant amount of glucose entry is facilitated by the insulin-dependent GLUT4 transporters.
Insulin does not only prompt the transfer of GLUT4 transporters from inside the cell to the cell membranes, but it also encourages the production of GLUT4. Consequently, insulin plays a significant role in managing the primary route for glucose entry into the cells.
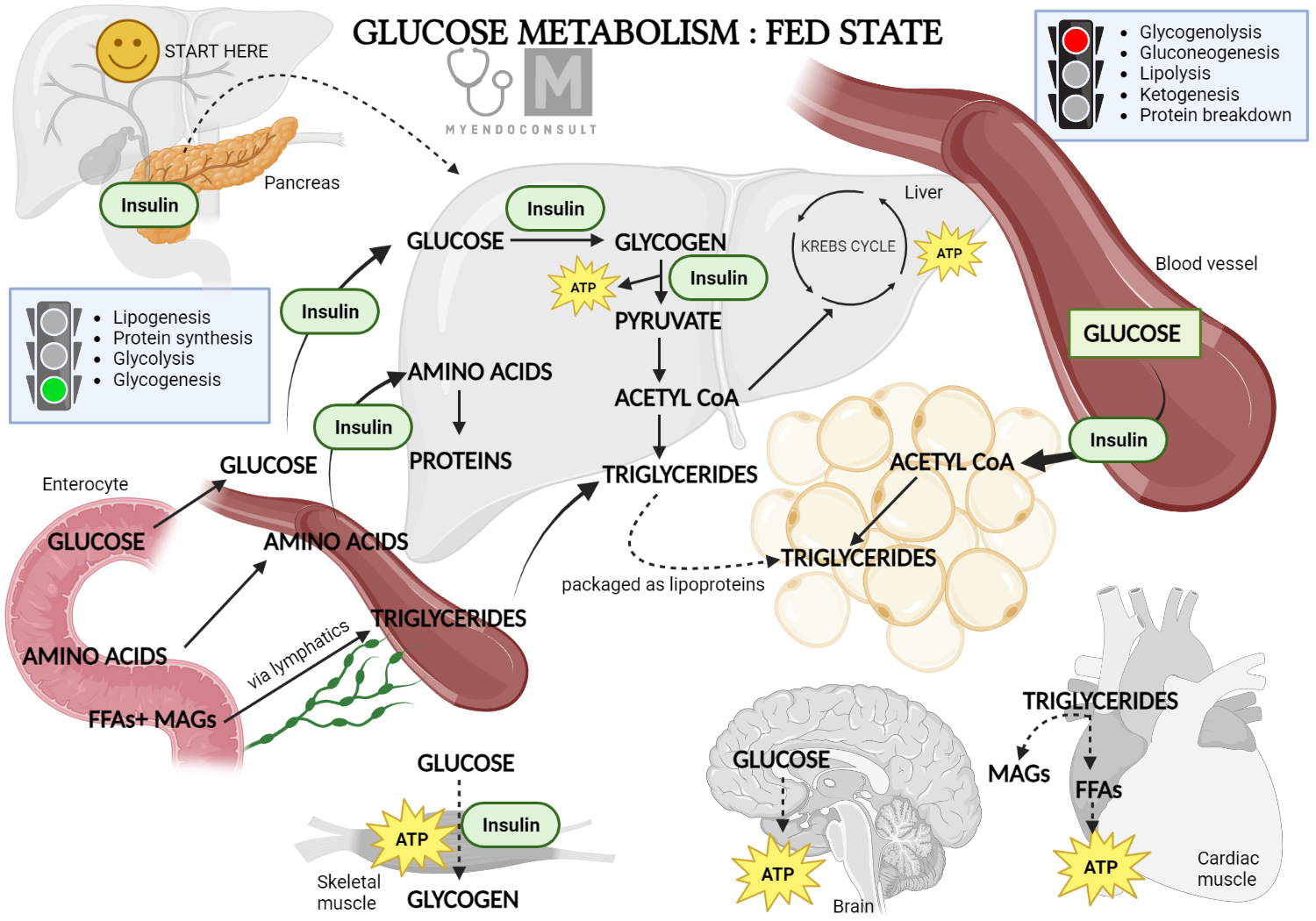
Role of insulin in glucose metabolism (fed state)
Impact on Glycolysis
Insulin also promotes the process of glycolysis within the cells, a metabolic pathway that allows cells to extract energy from glucose. Insulin achieves this by encouraging the production of crucial enzymes involved in glucose metabolism, such as glucokinase, phosphofructokinase, and pyruvate kinase. This activation occurs in all cells impacted by insulin, including muscle and fat cells.
Role in Glycogenesis
As glucose enters a cell, it is quickly converted to glucose-6-phosphate and either utilized instantly via the glycolytic pathway or stored in the form of glycogen. This process of transforming glucose-6-phosphate into glycogen (a process known as glycogenesis) is facilitated by insulin. Notably, this function of insulin extends to all cells, including liver cells, a major glycogen reservoir in the body.
Inhibiting Glycogenolysis
Glycogen, the intracellular form of glucose, is made available for glycolysis via a process called glycogenolysis. This is triggered by hormones such as adrenaline or glucagon when necessary. The glucose storage in the liver is particularly crucial, as upon glycogenolysis stimulation, it is released into general circulation, thereby becoming available for use by other cells. Insulin aids in regulating blood glucose levels by inhibiting the enzymes involved in glycogenolysis.
Gluconeogenesis and Insulin's Role
Insulin plays a crucial role in gluconeogenesis, a process where the body creates glucose from non-carbohydrate sources such as certain amino acids or glycerol. This process mainly occurs in the liver. Insulin inhibits gluconeogenesis, thereby preventing a potential increase in blood glucose concentration from this process.
Regulating Glucagon Production
Insulin indirectly impacts glucose production by curbing the release of glucagon, another hormone that raises blood glucose levels. This is primarily attributed to a paracrine effect. However, in conditions like diabetes mellitus where insulin levels are low or absent, one might expect high glucose levels to suppress glucagon levels. Surprisingly, glucagon levels often turn out higher than anticipated. This anomaly is possibly due to the lack of insulin's inhibitory effect on α-cells, cells in the pancreas that produce glucagon.
Insulin's Impact on Protein Metabolism
Insulin is also recognized as an anabolic hormone due to its role in promoting protein synthesis. It impacts both the uptake of amino acids into the cells and the actual synthesis of protein.
Boosting Amino Acid Uptake
Several transporters capable of facilitating the movement of amino acids across cell membranes have been identified, with some of them being responsive to insulin. For instance, the cationic amino acid transporter (CAT1) for the amino acid arginine and the transporter for alanine are both directly activated by insulin.
Promoting Protein Synthesis
Insulin not only likely boosts protein synthesis through its influence on amino acid transporters in the cell membrane but also stimulates new protein synthesis through an indirect genomic effect. Hence, insulin plays a significant role in normal body growth and development.
This role of insulin becomes evident in the accelerated growth observed in newborns of untreated diabetic mothers. This is primarily because the mother's elevated blood glucose level increases the transfer of glucose to the fetus through the placenta. The fetus's islets of Langerhans detect this increase and respond by boosting insulin release. The insulin then exerts its typical effects, stimulating both glucose storage and protein synthesis, which contributes to growth.
Regulating Lipid Metabolism
Another key process where glucose can be stored involves its conversion to lipid in fat tissue, known as lipogenesis. As glucose enters the glycolysis pathway, it eventually transforms into pyruvate, which can further convert to acetyl co-enzyme A (acetyl CoA) in the cell cytoplasm. This acetyl CoA can then morph into fatty acids, facilitated by a group of enzymes called fatty acid synthase.
Three free fatty acids can bind with one molecule of glycerol to form a triglyceride, which is then transported and stored as fat. Insulin plays a key role in promoting the synthesis of fatty acids and the creation of triglycerides. These are deposited in various tissues, especially adipose tissue. Moreover, insulin also inhibits lipolysis, the process of breaking down triglycerides back into fatty acids and glycerol, once again serving as a glucose storage hormone.
Understanding Ketogenesis
Acetyl co-A can also convert to acetoacetyl co-A in fat cells, especially when lipolysis is triggered in the absence of insulin. Acetoacetyl co-A then transforms into hydroxyl-β-methylglutaryl co-A, which further converts into acetoacetic acid. This acid and its two metabolites, acetone and β hydroxybutyric acid, are termed the ketone bodies.
Typically, in the presence of glucose, insulin suppresses the formation of ketone bodies. However, when insulin is barely present or absent, such as in type 1 diabetes, the concentration of ketone bodies in the blood increases. These acidic molecules can disrupt cell function as they lower pH levels.
The scent of acetone, often compared to that of pear drops or nail polish, can be a telling sign of type 1 diabetes. If detected on a person's breath, it may suggest a type 1 diabetes diagnosis, not excessive alcohol consumption!
Insulin's Role in the Brain
Specific neurons in the brain, notably in the hypothalamus, are highly sensitive to changes in glucose concentration in their surroundings. Depending on the glucose concentration, certain neuron groups may be inhibited while others stimulated. Many of these neurons express neuropeptides like orexin, melanin-concentrating hormone (MCH), and neuropeptide Y, which are thought to be involved in the regulation of energy balance by affecting food intake.
Insulin receptors, found throughout the central nervous system (CNS) and particularly in the hypothalamus, contribute to eating and satiety regulation. Given that circulating insulin levels rise after a meal, it is likely that insulin connects to the neural circuitry that signals fullness and reduced food intake under normal circumstances. The central regulation of food intake is a complex process involving multiple factors
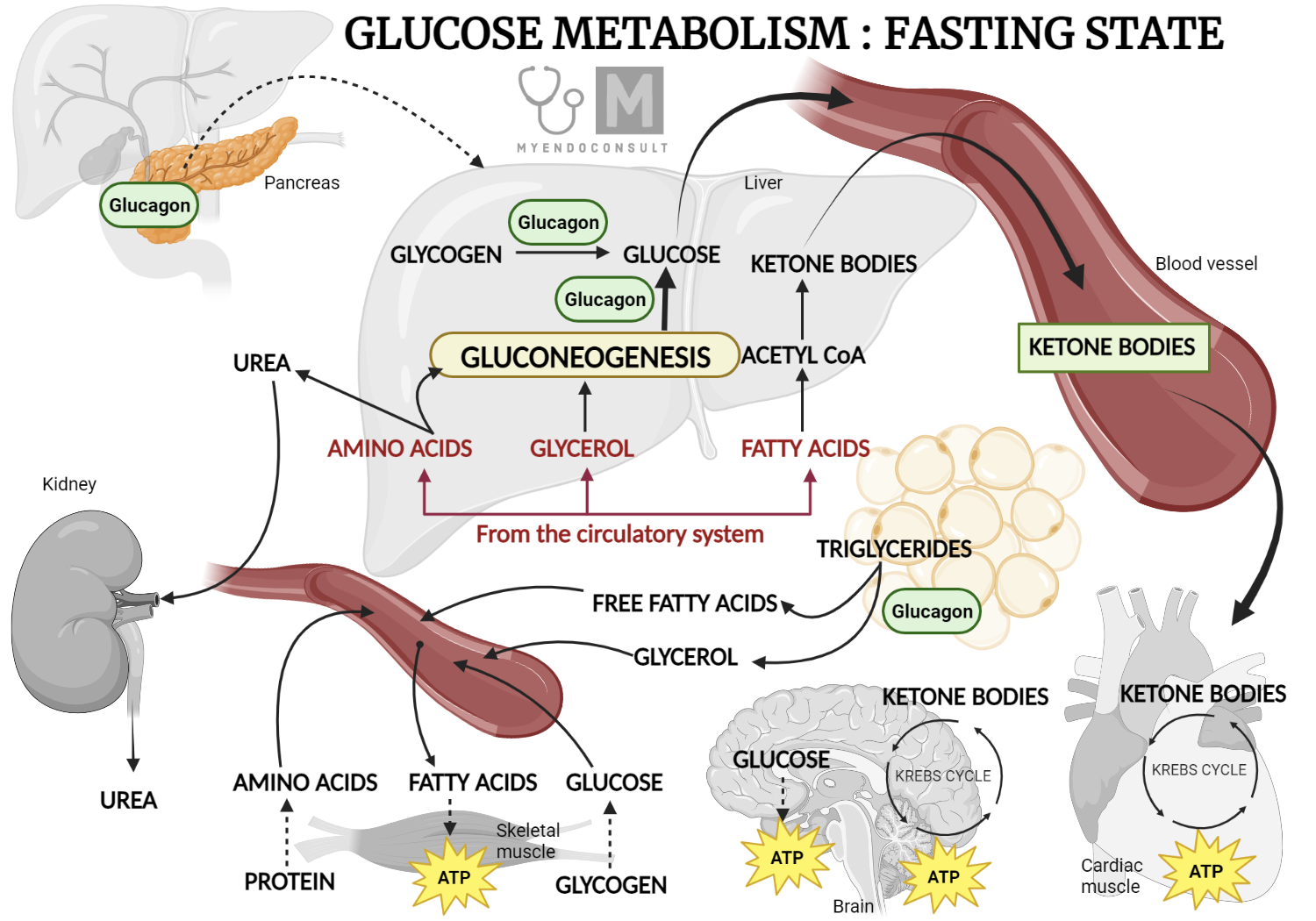
Role of glucagon in glucose metabolism (fasting state)
Understanding Glucagon's Role
Glucagon plays a crucial role in glucose metabolism, ultimately increasing the concentration of glucose in the blood. In this way, glucagon's effect is opposite to that of insulin. Its primary target is the liver, where it triggers the breakdown of glycogen (glycogenolysis) and the creation of glucose from non-carbohydrate sources (gluconeogenesis). Furthermore, glucagon also promotes the breakdown of triglycerides into fatty acids in adipose tissue, causing an increase in glycerol production, which then contributes to gluconeogenesis in the liver. This process provides an easily accessible source of glucose for other cells in the body.
Much like insulin, glucagon release is closely tied to changes in blood glucose levels. When blood glucose levels decrease, such as during fasting, glucagon is released. Intriguingly, certain gastrointestinal hormones that stimulate insulin release, like cholecystokinin, also trigger glucagon release. The autonomic nervous system further influences glucagon release, with increased sympathetic activity associated with a boost in glucagon release. This effect counteracts the inhibitory influence on insulin release.
Insulin itself also suppresses glucagon release, possibly through a paracrine effect, which likely explains why glucagon levels in untreated diabetic patients are often higher than expected given their elevated blood glucose levels - the restraining effect of insulin on glucagon release is diminished or absent. Additionally, somatostatin from δ-cells in the pancreas inhibits glucagon release, mirroring its action on insulin release.
Kindly Let Us Know If This Was helpful? Thank You!


