
Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure.
Review study protocol
Read Study Publication
Rationale and Clinical Equipoise
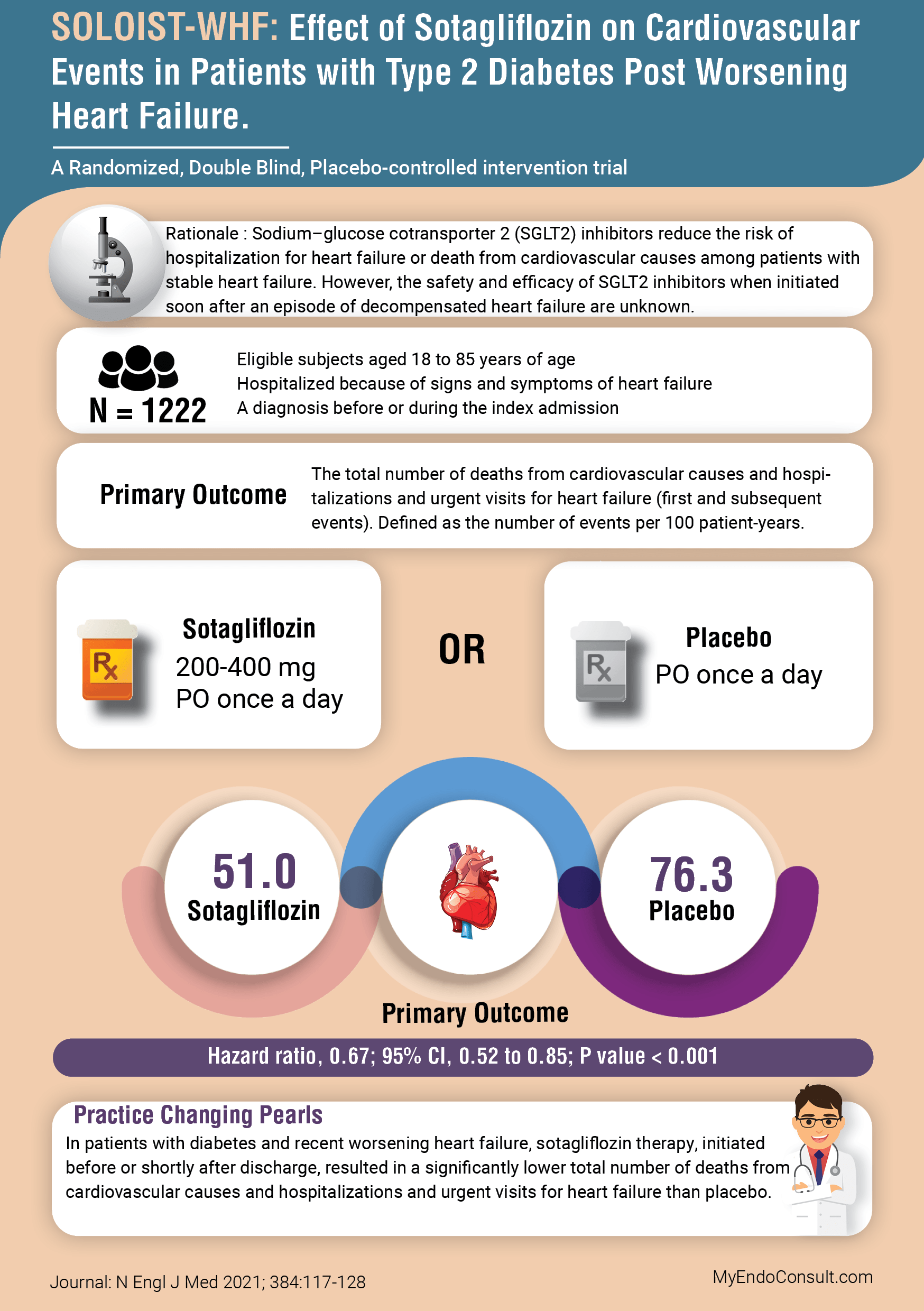
Sotagliflozin inhibits both SGLT2 receptors in the kidney and SGLT1 receptors in the intestine. Sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of hospitalization for heart failure or death from cardiovascular causes among patients with stable heart failure. However, the safety and efficacy of SGLT2 inhibitors when initiated soon after an episode of decompensated heart failure are unknown.
In addition, the potential deleterious effects of SGLT2 inhibitors including hypotension, volume depletion and consequent acute kidney injury in heart failure patients was considered by study investigators.
Study Design
- A randomized, double-blind, placebo-controlled phase III clinical trial
- 608 subjects were randomized to the intervention (sotagliflozin) arm and 614 to the placebo arm.
- An intention-to-treat analysis
- This study was terminated early due to lack of funds from the study sponsor.
Study Eligibility Criteria
- Eligible subjects aged 18 to 85 years of age
- Hospitalized because of signs and symptoms of heart failure
- A diagnosis before or during the index admission
- Type 2 diabetes mellitus
- Clinically stable (Not on oxygen therapy, Systolic blood pressure >100mmHg and not on intravenous inotropic or vasodilator medications (except nitrates)
Primary Outcome
A composite of total number of deaths from cardiovascular causes, hospitalizations and urgent visits for heart failure (first and subsequent events).
The primary endpoint comparing the sotagliflozin arm to the placebo arm, was 51.0 versus 76.3 events per 100 patient-years (Hazard ratio, 0.67; 95% CI, 0.52 to 0.85; P value < 0.0009)
Critical Appraisal
- As expected, there was a higher rate of fluid losses (gastrointestinal and renal) and hypoglycemia in the intervention arm. This could limit its application in the general population of diabetic patients with acute decompensated heart failure.
- Inadvertent early termination of the study may have underpowered it for more robust conclusions regarding the primary study outcome.
Practice Changing Pearls (Conclusion)
In patients with diabetes and recent worsening heart failure, sotagliflozin therapy, initiated
before or shortly after discharge, resulted in a significantly lower total number of deaths from
cardiovascular causes and hospitalizations and urgent visits for heart failure than placebo.
References
- Bhatt DL et al: SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021 Jan 14;384(2):117-128. doi: 10.1056/NEJMoa2030183.
- Packer M et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020 Oct 8;383(15):1413-1424. doi: 10.1056/NEJMoa2022190.
Kindly Let Us Know If This Was helpful? Thank You!


