
The two-cell two gonadotropin theory is central to our understanding of ovarian steroidogenesis. The origin, simplified model, and intracellular signaling mechanisms involved in this pathway will be described.
Origin of the two cell two gonadotropin hypothesis
The classic two cell, two gonadotropin model of estrogen synthesis was first proposed by Armstrong et al. in 1979. This model is sometimes referred to as the Armstrong-Dorrington “two-cell” model. It posits that FSH receptors are exclusively on granulosa cells, whilst LH receptors are expressed primarily by theca cells of the ovary.
In essence, stimulation of FSH receptors present on granulosa cells stimulates aromatase activity – promoting the conversion of androgens (androstenedione and testosterone) into estrogens1. Furthermore, the stimulation of LH receptors (present on theca cells) leads to their increased production of androgens. With granulosa and theca cells lying close to each other, there are additional paracrine mediators involved in the synthesis of estrogen.
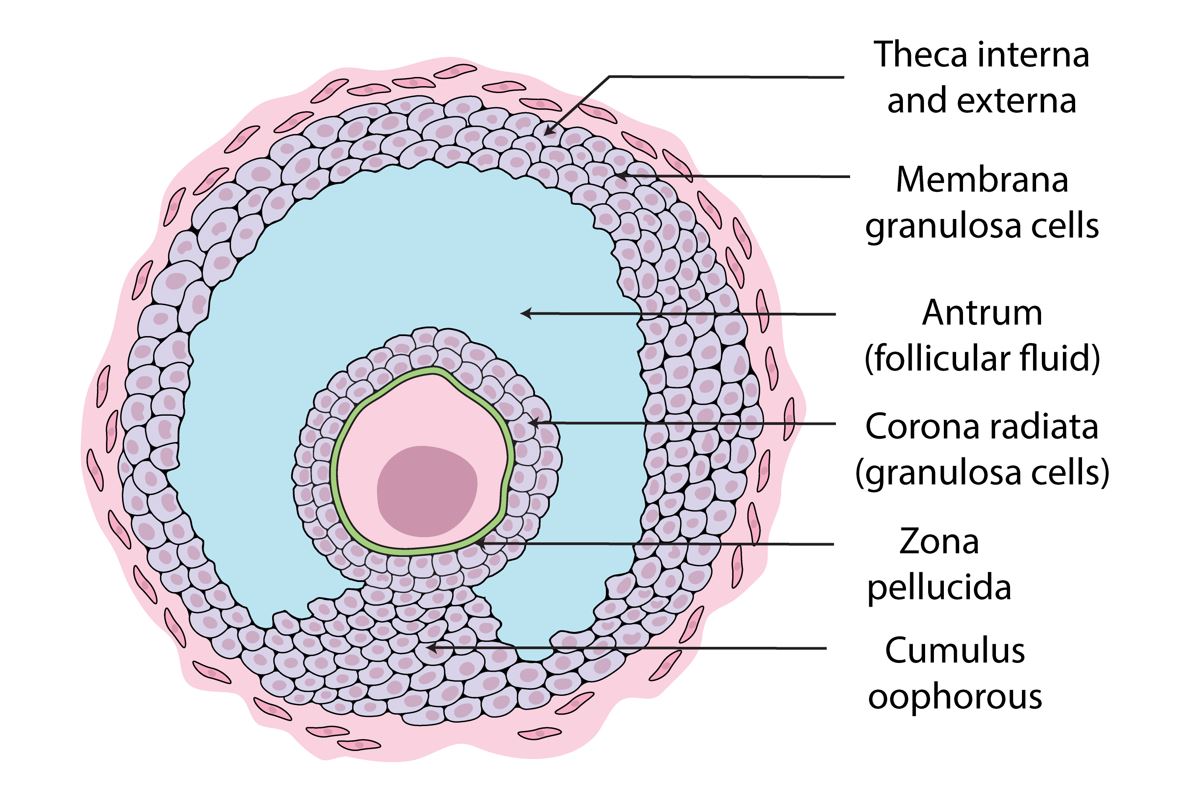
A simplified Model of Estrogen biosynthesis
In normal physiology, the synthesis of androgens by theca interna cells is induced by luteinizing hormone. Subsequently, androgens are shuttled to granulosa cells. Follicle-stimulating hormone then promotes aromatase enzyme activity in granulosa cells. This oversimplified model is referred to as the two-cell, two-gonadotropin theory of estrogen biosynthesis2.
Intracellular signaling of estrogen biosynthesis
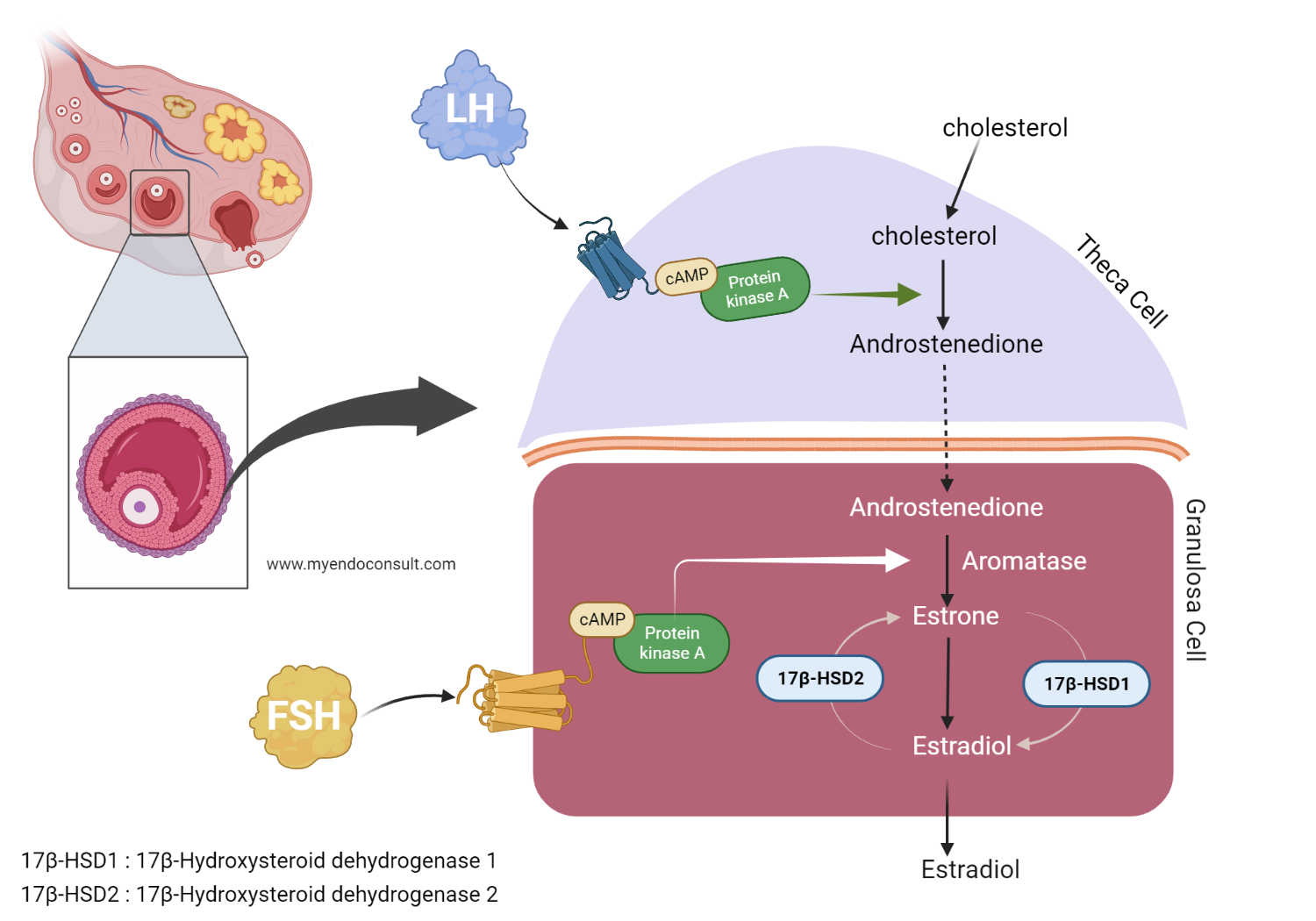
Firstly, luteinizing hormone by binding to its cognate receptor, the luteinizing hormone receptor (G-protein coupled receptor) promotes the production of intracytoplasmic cyclic adenosine monophosphate (cAMP) by the theca cell. Subsequently, cyclic adenosine monophosphate activates protein kinase A (PKA), which then induces the transcription of translation of various steroidogenic enzymes involved in androgen synthesis (testosterone, androstenedione, and dehydroepiandrosterone).
Likewise, FSH also stimulates its corresponding G-protein coupled receptor present on granulosa cells, thus increasing intracytoplasmic cAMP. Cyclic AMP then promotes the activity of PKA. Previously synthesized androgens from theca cell are then ferried to the granulosa cell. Aromatase activity in the granulosa (potentiated by PKA) promotes the conversion of androgens to estrogens (estradiol and estrone)3,4.
References
- Hillier, S. G., Whitelaw, P. F. & Smyth, C. D. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’model revisited. Mol. Cell. Endocrinol. 100, 51–54 (1994).
- Liu, Y.-X. & Hsueh, A. J. W. Synergism between Granulosa and Theca-Interstitial Cells in Estrogen Biosynthesis by Gonadotropin-Treated Rat Ovaries: Studies on the Two-Cell, Two-Gonadotropin Hypothesis Using Steroid Antisera1. Biol. Reprod. 35, 27–36 (1986).
- McNatty, K. P., Makris, A., DeGrazia, C., Osathanondh, R. & Ryan, K. J. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J. Clin. Endocrinol. Metab. 49, 687–699 (1979).
- Knecht, M., Amsterdam, A. & Catt, K. The regulatory role of cyclic AMP in hormone-induced of granulosa cell differentiation. J. Biol. Chem. 256, 10628–10633 (1981).
Kindly Let Us Know If This Was helpful? Thank You!


