Vitamin D, a critical fat-soluble vitamin, is integral to numerous physiological processes, with a central role in the regulation of calcium homeostasis and bone health. In addition to its established role in bone physiology, increasing research evidence indicates its involvement in various other aspects of human health, such as immune function, cardiovascular health, and cancer prevention, making accurate assessment of vitamin D status in individuals increasingly essential.
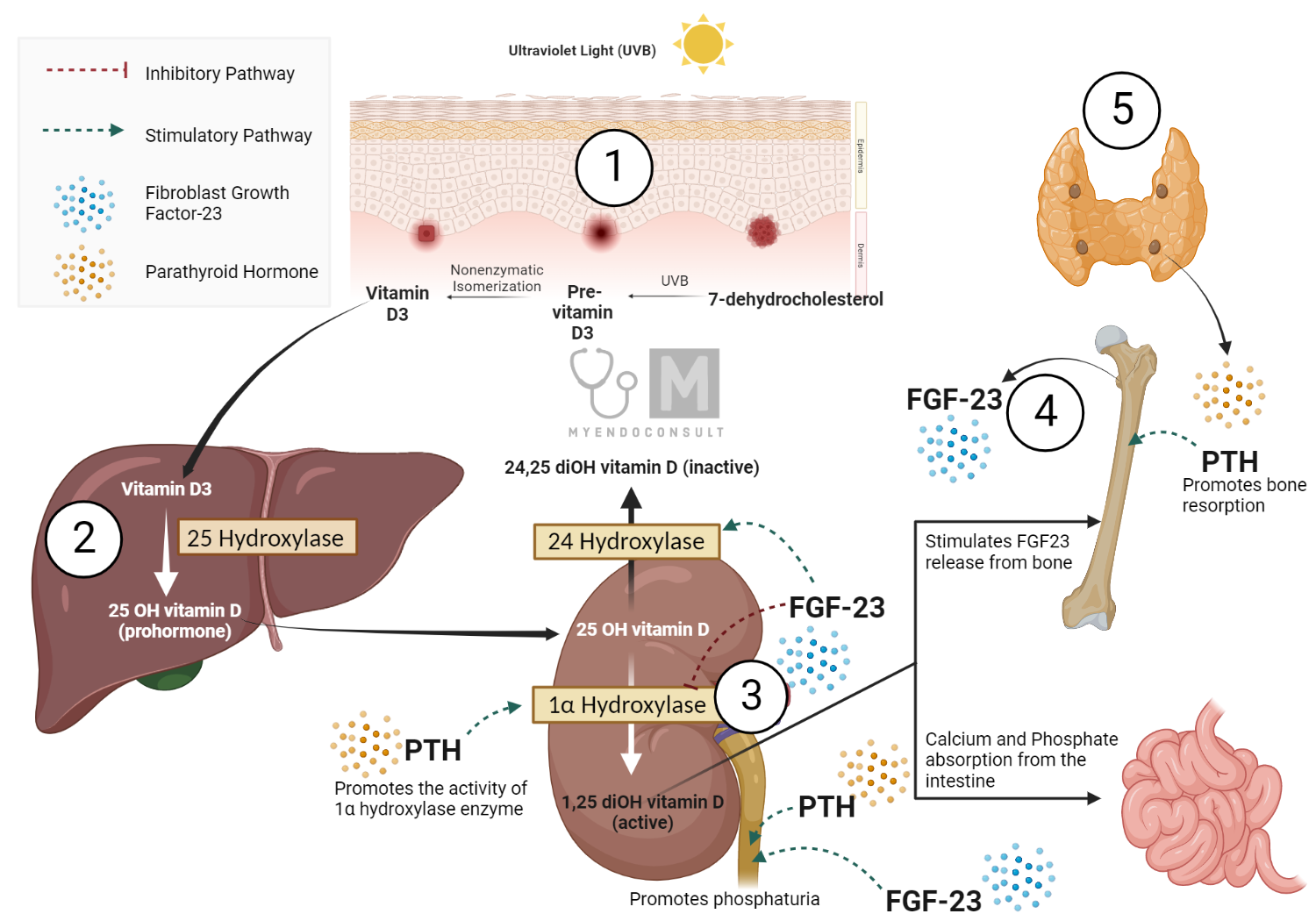
Vitamin D is unique in that it can be synthesized endogenously in the skin in response to exposure to sunlight, as well as being obtained from dietary sources and supplements. It exists in two primary forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). These forms are metabolized in the liver and kidneys into their active metabolites, including 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D], respectively, which are typically measured to determine an individual’s vitamin D status. Additionally, additional metabolites such as 24,25-dihydroxyvitamin D [24,25(OH)2D] are receiving increasing attention for their potential physiological functions.
Considering the importance of vitamin D and the implications of its deficiency, it is crucial to possess robust and reliable methodologies to measure the levels of this vitamin and its metabolites. Over the years, several assay methods have been developed, each with its own advantages and limitations. This article aims to present a comprehensive overview of the current methods used for vitamin D and its metabolites’ measurement, discussing their principle, sensitivity, specificity, and potential challenges.
Timeline of the Discovery of assay methods for vitamin D
The history of the discovery and measurement of vitamin D and its metabolites has seen continuous progress since the early 20th century. The initial recognition of vitamin D came through its connection with the prevention of rickets, a debilitating disease that affects mainly children. During this period, the primary method of detecting vitamin D was through biological assays that used its curative effect on rickets[1].
In the mid-20th century, advances in chemical isolation techniques led to the identification of key metabolites of vitamin D. These metabolites, 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D), were found to be crucial to understanding the biological function and regulation of vitamin D in the body.
The development of modern assay methods began in earnest in the 1960s and 1970s with the introduction of radioimmunoassay (RIA) and competitive protein-binding assays. These sophisticated techniques increased sensitivity and allowed the measurement of specific vitamin D metabolites in the blood. This period also saw the advent of high-performance liquid chromatography (HPLC) in the 1980s, which further improved the specificity of vitamin D assays by allowing the separation and measurement of individual metabolites.
The late twentieth century saw the development of immunoassays capable of detecting 1,25(OH)2D, despite its low concentration in the circulation. This technical advancement demonstrated the progression of measurement techniques in terms of sensitivity and specificity.
In the late 1990s and early 2000s, the field experienced the introduction of liquid chromatography tandem mass spectrometry (LC-MS/MS). This advanced technique further enhanced the accuracy and sensitivity of vitamin D measurements, marking a significant milestone in the field of vitamin D research.
As we enter the 21st century, the development of automated immunoassays makes vitamin D measurement more accessible and cost-effective for routine clinical use. Current research focuses on the development of rapid point-of-care testing methods for vitamin D. The aim of these developments is to make testing more widely available, thus broadening the ability to assess vitamin D status on a large scale. The journey from the early recognition of vitamin D to the development of sophisticated testing methodologies illustrates the evolving understanding and growing importance of this critical nutrient.
Advancements in Assays for Vitamin D and Its Metabolites
The accuracy and precision of assays to measure vitamin D2 and D3 (collectively referred to as vitamin D) and their metabolites in human serum or plasma have significantly advanced over the last decade or so.
The modern era of vitamin D metabolite assay technology was ushered in by the advent of competitive protein-binding assays and the realization that vitamin D could be metabolized into more active compounds. Two key technological advances that have greatly enhanced our ability to measure the less abundant metabolites of 25(OH)D are the development of high performance liquid chromatography (HPLC) for sample purification and the synthesis of radioligands of high specific activity.
Although the assay technology has progressed rapidly in terms of the vitamin D metabolites that can be measured, clinically useful measurements are currently limited to serum levels of 25(OH)D and 1,25(OH)2D.
Assay Techniques: Measurement of Vitamin D2 and Vitamin D3
The quantification of vitamin D2 and D3 in serum is a complex process, which, currently, does not yield significant clinically relevant information. Due to the lower polarity of Vitamin D2 and D3 compared to their hydroxylated metabolites, these two cannot be effectively purified or separated from each other using standard phase high performance liquid chromatography (HPLC).
Although sequential measurements of vitamin D3 concentration can potentially provide an indication of the amount of vitamin D3 synthesized in the skin after exposure to UV-B radiation, it does not adequately reflect the vitamin D status of the individual. This is illustrated by the fact that serum vitamin D concentrations in a group of healthy subjects, all of whom have a normal serum concentration of 25(OH)D, can fluctuate between summer and winter samplings. Rapid clearance of vitamin D from the serum results in a variable and unpredictable serum vitamin D concentration. Even in summer, levels may be low if the individual has not recently been exposed to substantial sunlight.
However, there is evidence to suggest that the serum vitamin D2 level could be a useful parameter in evaluating the sufficiency of absorption of an orally administered dose of 50,000 IU of vitamin D2. This could potentially offer a sensitive indicator of intestinal fat absorption.
The procedure for determining the concentration of 25(OH)D can vary, depending on the clinical situation and whether it is essential to understand the individual contributions of 25(OH)D3 and 25(OH)D2 to the total circulating concentration of 25(OH)D. In assays that do not use HPLC with direct UV absorbance for detection, a 25(OH)D concentration can be easily assessed.
All routine 25(OH)D assays, regardless of the detection method, require lipid extraction from the serum to release the compound from the protein constituents of the serum that bind to 25 (OH) D.
Lipoprotein extraction for the 25(OH)D assay can be performed using various solvents, including methanol, diethyl ether, or even absolute ethanol. The raw lipid extract can then be placed directly into a competitive protein-binding assay with a form of serum vitamin D-binding protein (DBP) and vitamin D3 as the displaceable ligand. Most 25(OH)D assays use whole serum from vitamin D-deficient rats as a source of binding protein. However, since only about 1% of the total binding sites on DBP are occupied by vitamin D or its metabolites in the vitamin D-sufficient state, serum from a vitamin D-replete animal can be used without significantly altering the sensitivity of the assay.
Measurement of 25(OH)D2 has some clinical importance in a patient consuming a multivitamin preparation containing vitamin D2. Since hepatic microsomal vitamin D-25-hydroxylase does not distinguish between vitamin D2 and vitamin D3 as substrates. Therefore, a low circulating concentration of 25(OH)D3 in such an individual can indicate that endogenous vitamin D3 synthesis is deficient and that the patient relies on dietary or therapeutic vitamin D2 to maintain an adequate vitamin D nutritional status. However, it should be noted that in the United States, foods are supplemented with vitamin D2 or vitamin D3, and measurement of 25(OH)D2 and 25(OH)D3 cannot be used as a method to determine the source of vitamin D.
Assay Techniques: Measurement of 1,25(OH)2D
The first serum assay for 1,25(OH)2D emerged in the 1970s. The development hinged on the identification of a high-affinity, low-capacity binding protein (receptor) in the chick intestinal epithelium that could be employed as a specific binding protein in a radioligand binding assay.
In recent times, advancements have been made because of the availability of tracers with high specific activity, the use of advanced chromatography for sample purification, and techniques to promote receptor stability. These enhancements have significantly improved sample preparation and analysis, making the 1,25(OH)2D assay widely available in most university hospitals and several commercial laboratories. Despite this relative ease in obtaining the 1,25 (OH) 2D assay in patients’ serum or plasma, variability in its performance exists, and there are considerations in its application to human health or disease.
A purified lipid extract of human serum or plasma can be analyzed for its 1,25(OH)2D content in several ways. The classical binding protein in the competitive protein-binding assay for 1,25(OH)2D is the hormone’s own receptor protein isolated from the intestinal epithelium of vitamin D-deficient chicks. Vitamin D-deficient birds are used to improve the extraction of receptors that are not occupied by endogenously synthesized 1,25(OH)2D[2].
One drawback of using the chick intestinal receptor in the binding assay is its discrimination against 1,25(OH)2D2 binding in favor of 1,25(OH)2D3. Consequently, these two metabolites, which are biologically equipotent in humans, cannot be measured with equal efficiency.
These challenges have been addressed with the increased use of the receptor derived from calf thymus, which binds 1,25(OH)2D3 and 1,25(OH)2D2 with equivalent affinity and capacity. Indeed, this assay can now be used for the measurement of 1,25(OH)2D in small quantities of human serum (0.5 to 1.0 ml) after purification in a single silica cartridge[3].
Several radioimmunoassays have been established for the detection of 1,25(OH)2D in serum. Despite their potential advantages, radioimmunoassays have two primary limitations. First, similar to the chick intestinal receptor, numerous antibodies raised against 1,25(OH)2D3 analogues bind less avidly to 1,25(OH)2D2 compared to 1,25(OH)2D3. Consequently, these radioimmunoassays struggle with accurately determining the contribution of 1,25(OH)2D2 to the overall concentration of 1,25 (OH) 2D. Second, antibodies raised against 1,25(OH)2D3 exhibit cross-reactivity with other vitamin D metabolites. The absence of an efficient immunogen probably explains why the successful development of a robust antibody against hormone has been elusive[4].
Assay Techniques: Measurement of 24,25(OH)2D
Measurement of 24,25-dihydroxyvitamin D, or 24,25-(OH)2D, can be conveniently achieved using a competitive protein binding assay, thanks to the high affinity of vitamin D binding protein (DBP) for this metabolite. However, 24,25(OH)2D2 is bound twice as avidly than 24,25(OH)2D3, complicating the estimation of the total concentration of 24,25 (OH) 2D, particularly in individuals ingesting significant amounts of vitamin D2. Therefore, an accurate estimate of the total 24,25(OH)2D concentration of 24,25 (OH) 2D requires the use of chromatographic techniques that can distinguish between 24,25(OH)2D2 and 24,25(OH)2D3, and binding assays that utilize the appropriate radioinert metabolite in a standard curve.
While the measurement of circulating 24,25(OH)2D concentration might be seen as labor-intensive, it is important to question whether such determination is necessary. Although several researchers have proposed a physiological role for 24,25(OH)2D in human mineral metabolism, such a role has not been unequivocally demonstrated. Even more challenging is the lack of a widely acceptable reference range for 24,25(OH)2D, making its clinical utility highly debatable[5].
Conclusion
Measurement of vitamin D and its metabolites has proven to be an essential component in the clinical and research spheres of health science, particularly given the multifaceted roles of vitamin D in human physiology. The ability to accurately determine vitamin D status in individuals is crucial, not only to diagnose and treating vitamin D deficiency-related conditions but also for further elucidating the myriad of functions that this essential nutrient performs in our bodies.
In this article, we have reviewed the existing assay methodologies for the quantification of vitamin D and its metabolites, demonstrating that each comes with its unique set of advantages and challenges. Although certain methods offer high specificity, others excel in terms of sensitivity or convenience. However, none of the available methods fully address the need for an assay that is simultaneously rapid, cost-effective, accurate, and applicable to large-scale screenings. The complexity of vitamin D metabolism, the existence of various forms and metabolites, and the differences in their biological activity further compound the challenge.
However, the ongoing advancements in biotechnology and analytical chemistry hold promise for the development of more refined assay methodologies. The pursuit of a ‘gold standard’ assay for vitamin D continues, and this journey is of utmost importance, as a comprehensive understanding of vitamin D physiology and pathophysiology is highly dependent on the precision and accuracy of these analytical tools.
Lastly, it is imperative to recognize that the value of vitamin D assays extends beyond the realm of clinical diagnostics. They also serve as fundamental tools that enable research into the broader roles of vitamin D in health and disease, thereby contributing to the advancement of preventive and personalized medicine.
References (Selected)
1. DeLuca, H.F. (2014) History of the discovery of vitamin D and its active metabolites. Bonekey Rep, 3, 479.
2. Hunziker, W., Walters, M.R., Bishop, J.E., and Norman, A.W. (1982) Effect of vitamin D status on the equilibrium between occupied and unoccupied 1,25-dihydroxyvitamin D intestinal receptors in the chick. J Clin Invest, 69 (4), 826–833.
3. Tsugawa, N., Nakagawa, K., Kurobe, M., Ono, Y., Kubodera, N., Ozono, K., and Okano, T. (2000) In vitro biological activities of a series of 2 beta-substituted analogues of 1 alpha,25-dihydroxyvitamin D3. Biol Pharm Bull, 23 (1), 66–71.
4. Garnett, E., Li, J., Rajapakshe, D., Tam, E., Meng, Q.H., and Devaraj, S. (2019) Efficacy of two vitamin D immunoassays to detect 25-OH vitamin D2 and D3. Practical Laboratory Medicine, 17, e00130.
5. Dirks, N.F., Ackermans, M.T., Jonge, R. de, and Heijboer, A.C. (2019) Reference values for 24,25-dihydroxyvitamin D and the 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio. Clinical Chemistry and Laboratory Medicine (CCLM), 57 (10), e259–e261.
Kindly Let Us Know If This Was helpful? Thank You!


