I. Overview Of Cushing's Disease
- Cushing's disease is caused by a pituitary tumor that produces excessive, uncontrolled amounts of ACTH, leading to a rise in cortisol levels.
- This is specific to a tumor of the corticotrophs of the pituitary gland, leading to a disruption in ACTH regulation and a loss of the diurnal rhythm.
II. Clinical Features Of Cushing's Disease
- Patients experience a gradual increase in appetite and weight gain due to increased cortisol levels.
- Fat synthesis is promoted over protein synthesis, resulting in muscle weakness and a condition known as proximal myopathy.
- Skin healing becomes poor, making wounds to take longer to heal.
- Rapid increase in girth combined with reduced protein synthesis leads to stretch marks on the skin.
- Patients may develop a moon-shaped facial appearance due to increased fat deposition, thin skin with poor wound healing, an interscapular fat pad known as a ‘buffalo hump’, difficulty climbing stairs due to proximal myopathy, osteoporosis, and hypertension.
III. Diagnosis Of Cushing's Disease
- Random cortisol measurement is not helpful in diagnosis, as the total exposure of the patient to glucocorticoids increases even if the plasma cortisol level during the day may be within normal limits.
- The optimal time to measure cortisol concentration is when it is expected to be low, such as at midnight in a sleepy patient. However, it can be challenging to ensure that the patient is asleep.
- To confirm the diagnosis, other tests are utilized, the most popular being the dexamethasone suppression test. In this test, oral dexamethasone (a synthetic glucocorticoid) is administered to suppress the hypothalamo-pituitary-adrenal axis (HPA), and failure to suppress suggests Cushing's disease.
IV. Treatment Of Cushing’s Disease
Pasireotide
Pasireotide is a "near pan-somatostatin" receptor analog because it binds to four of the five isoforms of the somatostatin receptor (SSR1, SSR2, SSR3 and SSR5). In fact, pasireotide binds to the SSR5 receptor subtype more avidly than other somatostatin receptors, thus its demonstrable efficacy in Cushing's disease. Corticotroph tumors in the anterior pituitary gland express more SSR5 receptors than other subtypes of somatostatin receptors. Furthermore, cortisol negative feedback inhibition of somatostatin receptor expression by corticotrophs tends to impact SSR2 receptors more than the SSR5 receptor subtype. Due to its affinity for SSR5 receptors, pasireotide is an ideal therapeutic option for Cushing's disease[1].
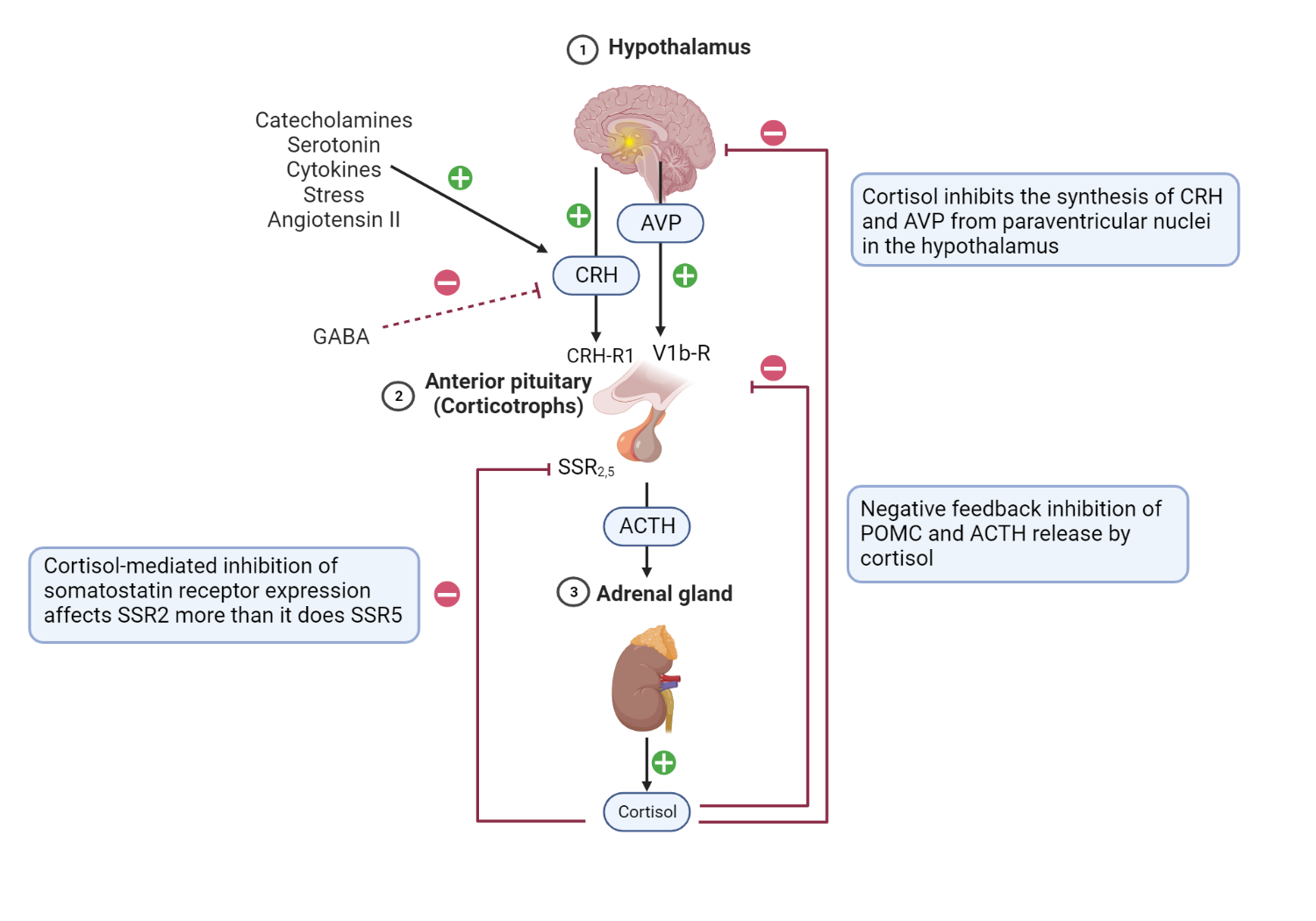
Schematic representation of the HPA axis, highlighting critical stimulatory and inhibitory feedback loops.
Hypothalamic and pituitary processes: CRH is released under trophic stimulation by various factors, including catecholamines, angiotensin II, serotonin, stress, and cytokines[2]. On the contrary, GABA inhibits CRH release and ultimately ACTH production of ACTH[3]. CRH from the hypothalamus stimulates anterior pituitary corticotrophs to release their preformed ACTH from secretory vesicles (fast response). Furthermore, CRH increases the expression of the POMC gene by anterior pituitary corticotrophs (slow response).
Furthermore, PVN AVP binds to V1b receptors in corticotrophs, further enhancing the action of CRH at the level of the anterior pituitary gland[4]. Activation of dopamine D2 receptors (D2R) present on corticotroph cells by hypothalamic-derived dopamine inhibits ACTH synthesis and release (not shown) [5]. Adrenal cortex processes: Binding of ACTH to the MCR-2 receptors present in cells in the zona fasciculata promotes the synthesis of cortisol from cholesterol[6]. Feedback Inhibitory Process: Negative feedback inhibition of POMC and ACTH release is mediated by adrenal-derived cortisol[7]. Furthermore, cortisol inhibits the synthesis of CRH and AVP from paraventricular nuclei in the hypothalamus[8]. Cortisol-mediated inhibition of somatostatin receptor expression in corticotrophs affects SSR2 more than it does SSR5[9]. + = shows stimulatory factors and feedback loops, and - = shows inhibitory factors and feedback loops.
The typical dose range for immediate-release pasireotide is 0.3 to 0.9 mg (300-900 mg) as a subcutaneous injection (thigh, upper arm or abdomen) twice daily. A long-acting release (LAR) formulation is administered once a month (10 to 30mg) intramuscularly as a depot injection by a health worker[10]. In practice, the LAR formulation is introduced after patients have demonstrated a response to immediate release of pasireotide.
Retinoic Acid
Retinoic acid (RA) reduces cortisol synthesis in subjects with Cushing disease through various observed mechanistic pathways.
RA reduces the synthesis of ACTH and POMC in corticotroph tumors[11]. This was reviewed earlier.
In addition, RA has direct tumoricidal effects on corticotroph tumors[11].
- RA reduces adrenal cortisol production through its antiproliferative effects on hyperplastic adrenocortical cells[11].
- RA also down-regulates the expression of the melanocortin-2 receptor (MCR-2) by adrenal cells[12].
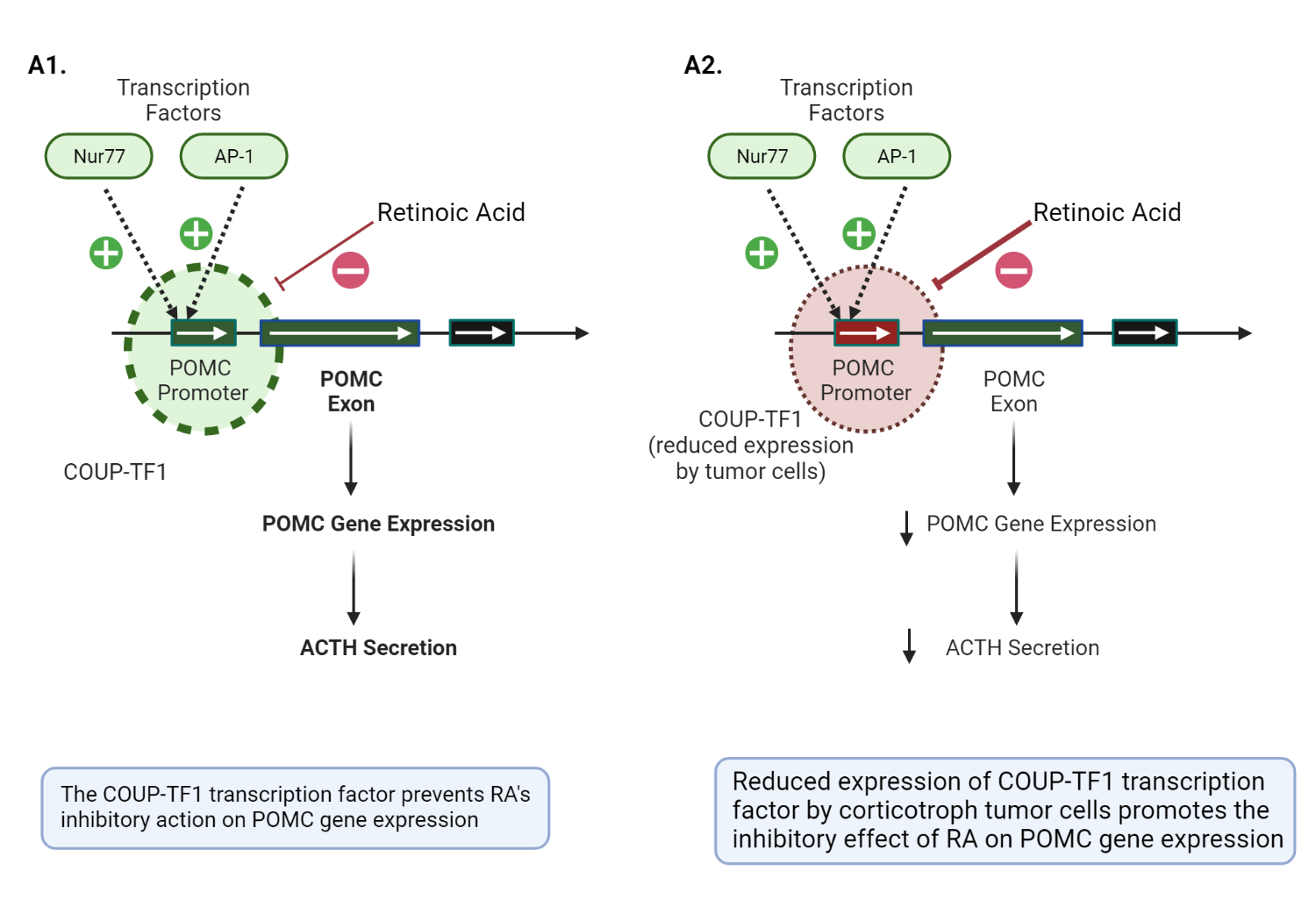
The role of retinoic acid in the pathophysiology of anterior corticotrophs.
AP-1 and Nur77 are critical transcription factors required for the activation of the POMC promotor gene in a corticotroph cell. The COUP-TF1 transcription factor prevents the inhibitory action on both AP-1 and Nur77, allowing POMC transcription and ACTH secretion to proceed in normal physiology(image A1)[13, p. 11]. In contrast, corticotroph tumors have a reduced expression of COUP-TF1, which allows RA to inhibit AP-1 and Nur77 (critical mediators of POMC promotor gene activation) (image A2)[11]. The thickness of the dashed circle = degree of expression of COUP-TF1. Adapted and modified from Pecori GF et al. (2012) Potential role of retinoic acid in patients with Cushing's disease. J Clin Endocrinol Metab 97:3577–3583[11]
Retinoic acid is teratogenic and should be used with extra caution in women of the reproductive age group. Other reported side effects of RA include photosensitivity and mucositis[14].
RA receptor activation increases the cortisol suppressive effects of dopaminergic agonists (DAs). Combining RA and DA is a suggested therapeutic option in patients with Cushing's disease[15].
Dopaminergic Agonists
Although approximately 80% of corticotroph adenomas express D2 receptors, they have relatively low density of D2 receptors, making dopaminergic agonists (DA) a less favorable therapeutic option[16]. Bromocriptine and cabergoline reduce cortisol production by binding to D2 receptors present in corticotrophs; however, they are not as effective as SSA [5].
Patients taking dopaminergic agonists exhibit an "escape phenomenon," which is characterized by up to a third of patients who responded previously experiencing rebound hypercortisolemia[16].
Cabergoline is associated with valvular heart disease, especially in patients exposed to doses close to the upper limit of the acceptable dose range[17]. Therefore, serial echocardiograms are reasonable in patients taking high doses of cabergoline[17] or exposed to a cumulatively high lifetime dose[18].
Common side effects of dopaminergic agonists include postural dizziness, nausea, and headaches[19].
In contrast to the lower dose range of 0.5 to 2.0 mg per week of cabergoline used in prolactinomas[20], a much higher dose range between 2.5 and 5 mg per week is required to treat CD[21,22].
Steroidogenesis Inhibitors
The mechanism of action of metyrapone, mitotane, ketoconazole, and the recently approved steroidogenesis inhibitor, osilodrostat, will be reviewed next.
Metyrapone: Metyrapone has a pyridine moiety, which allows it to alter the activity of 11beta-hydroxylase (critical in the final step of cortisol synthesis). Other metyrapone-inhibited steroidogenic enzymes include the 17 alpha-hydroxylase(17α-OH) and 18-hydroxylase enzymes (less potent inhibition)[23].
Mitotane: Mitotane is suggested to have both "adrenolytic" (adrenal cell death) and "adrenostatic" (enzymatic inhibition) properties. Mitotane is a chemotherapeutic agent with a diphenylmethane moiety that causes mitochondrial dysfunction, lysis, and necrosis. Mitotane, as stated above, exerts its "adrenostatic" function by inhibiting the side chain cleavage enzyme, 11beta hydroxylase, and 3βHSD [23].
Ketoconazole: Ketoconazole has an imidazole group (confers its antifungal properties) with demonstrable inhibitory effects on various steroidogenesis enzymes (in particular, the side chain cleavage complex, 17 alpha-hydroxylase and 11 beta-hydroxylase) in the adrenal cortex and gonads[23].
Osilodrostat: Osilodrostat, like metyrapone, inhibits 11-beta hydroxylase activity in the adrenal cortex. However, unlike metyrapone, osilodrostat has a relatively higher potency and a shorter plasma half-life; thus, it can be administered less frequently (two times a day instead of four times daily)[24].
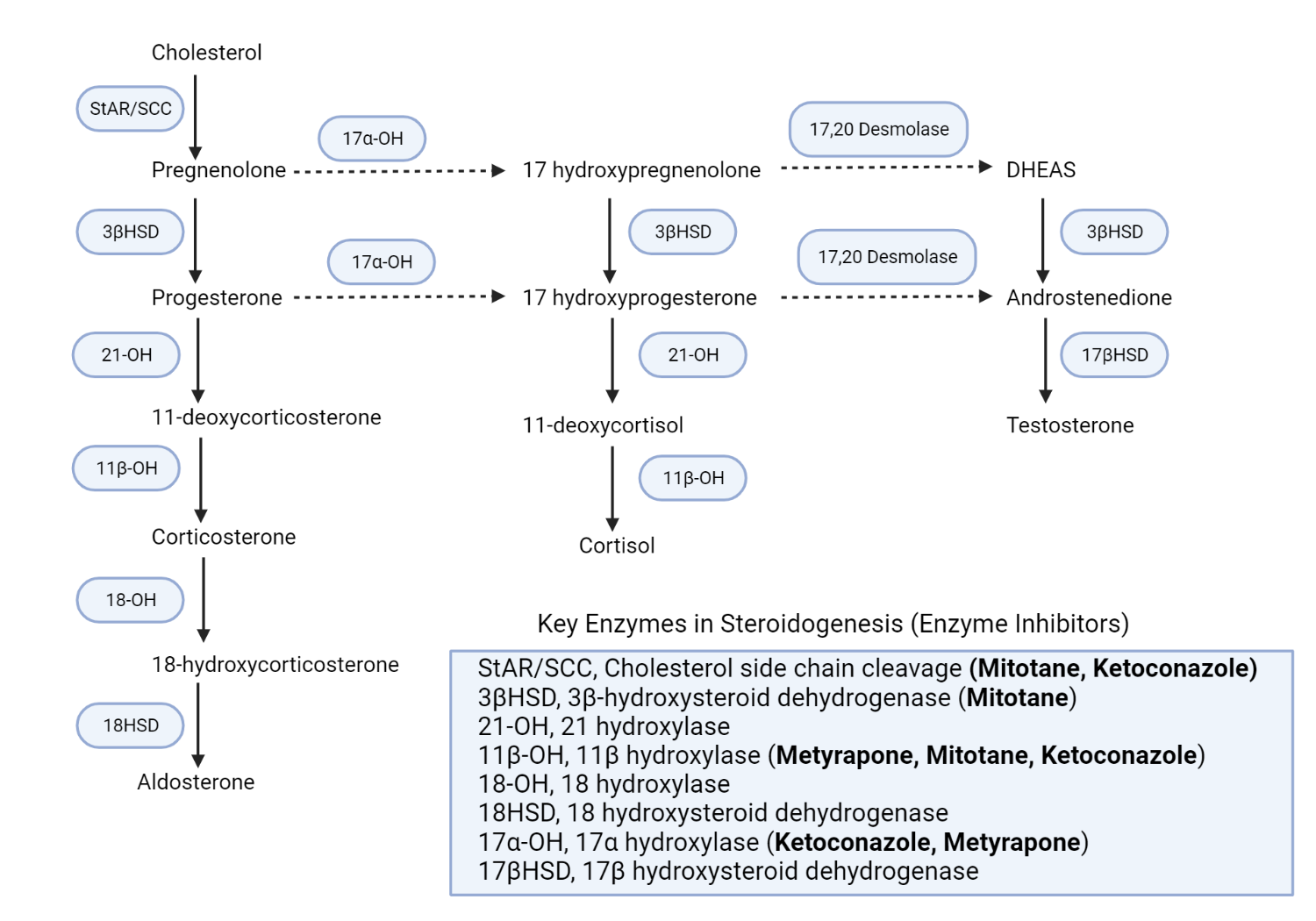
Schematic representation of the adrenal steroidogenesis pathways and the site of action of various enzyme inhibitors.
Metyrapone primarily inhibits the activity of 11 beta-hydroxylase activity (11β-OH), which results in a reduction in cortisol production of cortisol[25]. Consequently, there is an accumulation of intermediate mineralocorticoid precursors (11-DOC)[26] and a shunting of progesterone and pregnenolone to androgen production[27]. Mitotane inhibits various adrenal steroidogenic enzymes, including the side chain cleavage enzyme complex(StAR/SCC), 11beta hydroxylase(11β-OH), and 3βHSD[23]. Ketoconazole similarly inhibits various steroidogenic enzymes, including the side chain cleavage enzyme complex, 17alpha hydroxylase (17α-OH), and 11β-OH enzymes[23]. Redrawn and modified from Daniel E, Newell-Price JDC (2015) Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing syndrome. Eur J Endocrinol 172:R263-280[28]
Ketoconazole-induced apparent mineralocorticoid excess
There is a reported case of apparent mineralocorticoid excess (AME) in a patient treated with ketoconazole. The patient developed hypokalemia and hypertension in a manner that simulated a "non-glucocorticoid-mediated" AME. Unlike metyrapone, which mainly inhibits 11-beta hydroxylase (not to be confused with 11β-HSD), ketoconazole inhibits 11-beta hydroxylase and proximal side chain cleavage enzymes. Conventionally, this results in a relatively lower concentration of 11-Deoxycorticosterone (a potent mineralocorticoid) in patients treated with ketoconazole compared to metyrapone. Therefore, hypertension and hypokalemia can be inadvertently attributed to refractory hypercortisolemia (effects of excess cortisol overwhelming 11β-HSD2 and activating renal MCR) rather than accumulation of DOC (see Figure 1.4). In this scenario, an increase in the dose of ketoconazole due to the presumed uncontrolled hypercortisolemia would exacerbate hypertension and hypokalemia (mineralocorticoid effects of DOC). Although DOC-mediated hypertension is more common in patients treated with metyrapone, this rare case report highlights the possibility of ketoconazole-mediated hypertension due to accumulation of DOC in Cushing's syndrome[29].
Mifepristone
Mifepristone, also known as RU486 (Roussel-Uclaf 38486), has antiglucocorticoid and antiprogesterone effects. RU486 binds to the ligand-binding domain of the cytosolic glucocorticoid receptor without directly promoting its downstream effects, such as the activation of hormone response elements and transcription factors involved in glucocorticoid-mediated gene function[30].
There are two isoforms of the glucocorticoid receptor (GR), namely, the GRα and GRβ glucocorticoid receptor. The GRα isoform is the proverbial "classic glucocorticoid receptor" in the cytosol, while the GRβ isoform resides in the nucleus. The GRβ receptor exerts a mainly modulatory role by inhibiting the function of GRα[31].
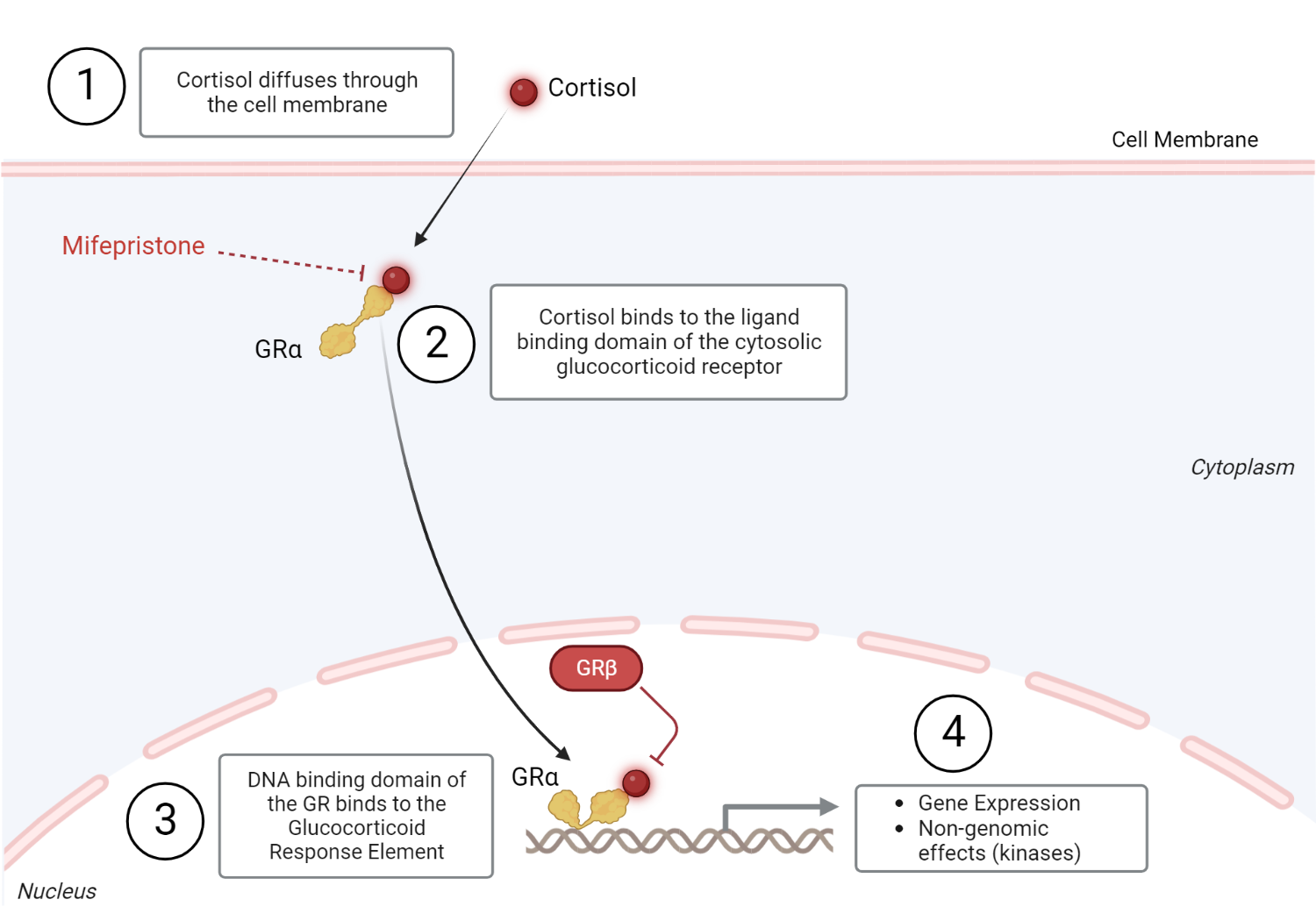
Glucocorticoid receptor physiology
Cortisol (glucocorticoid), a steroid hormone, diffuses through the plasma membrane to bind to the ligand-binding domain (LBD) of the cytosolic glucocorticoid receptor (Step 1). The hormone-receptor complex undergoes a conformational change and is then translocated from the cytosol to the nucleus to exert its genomic effects (step 2). The glucocorticoid receptor's DNA binding domain (DBD) binds to its assigned hormone response sequence, the Glucocorticoid Response Element (GRE), to exert its intranuclear effects. This leads to the activation of transcription factors that can activate or inactivate gene expression[31,32]. Glucocorticoids can also exert nongenomic effects through various kinases (step 3). This typically occurs minutes after ligand-receptor interaction in the cytosol[31]. The non-genomic effects of glucocorticoids include inflammatory and non-inflammatory processes (calcium mobilization and muscle function) processes[33]. GRβ inhibits the intranuclear effects of GRα[31]. Redrawn and modified from Oakley RH, Cidlowski JA (2013) The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J Allergy Clin Immunol 132:1033–1044[31]
Summary Of Therapeutic Targets In Cushing's Disease
Therapy | The primary site of action |
Pasireotide | Anterior pituitary gland (SSR1, SSR2, SSR3, and SSR5 receptors) |
Retinoic acid | Anterior pituitary gland (Retinoic acid receptors) |
Cabergoline | Anterior pituitary gland (Dopaminergic receptors) |
Ketoconazole | Adrenal steroidogenesis inhibitor |
Metyrapone | Adrenal steroidogenesis inhibitor |
Mitotane | Adrenal steroidogenesis inhibitor |
Mifepristone | Glucocorticoid receptor antagonist |
Adapted from Hinojosa-Amaya, JM et al. (2019), Medical Management of Cushing syndrome: Current and emerging treatments. Drugs 79:935–956 [34]
References
Kindly Let Us Know If This Was helpful? Thank You!


