The electron transport chain (ETC) represents the final stage of cellular respiration, a process that converts nutrients into usable energy for the cell. This biochemical sequence occurs within the mitochondria and is critical for energy production. This article provides a detailed look at the ETC, highlighting its components, mechanism, and importance in cellular energy metabolism.
Components of the Electron Transport Chain
The ETC consists of four multi-protein complexes located in the inner mitochondrial membrane: Complex I (NADH: ubiquinone oxidoreductase), Complex II (succinate: ubiquinone oxidoreductase), Complex III (cytochrome c: ubiquinol oxidoreductase), and Complex IV (cytochrome c oxidase).
Additionally, two mobile electron carriers participate in the chain: ubiquinone (coenzyme Q) and cytochrome c. A fifth complex, ATP synthase (Complex V), is not part of the ETC itself but is crucial for ATP production driven by the ETC.
Mechanism of the Electron Transport Chain
The primary role of the ETC is to create a proton gradient across the inner mitochondrial membrane. This gradient drives ATP synthesis in a process known as oxidative phosphorylation.
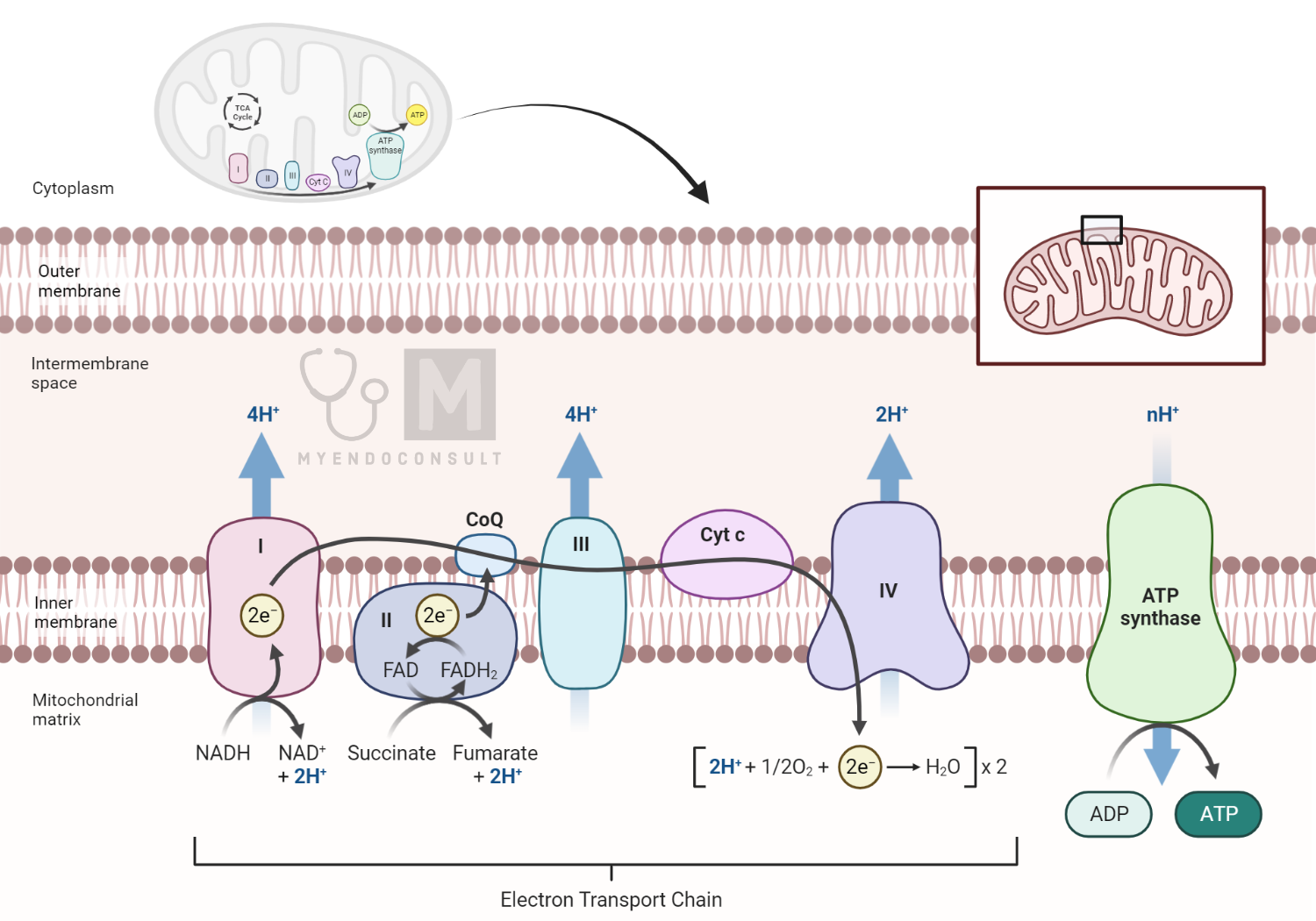
- Complex I: This is the entry point for electrons from NADH, a product of glycolysis and the citric acid cycle. Complex I transfers electrons from NADH to ubiquinone, pumping protons from the mitochondrial matrix to the intermembrane space in the process.
- Complex II: This complex adds electrons from another source – succinate, a product of the citric acid cycle. Unlike Complex I, Complex II does not pump protons across the membrane.
- Ubiquinone: This small hydrophobic molecule shuttles electrons from Complexes I and II to Complex III.
- Complex III: This complex transfers electrons from reduced ubiquinone to cytochrome c, simultaneously pumping more protons across the membrane.
- Cytochrome c: A small soluble protein, cytochrome c carries electrons from Complex III to Complex IV.
- Complex IV: Here, electrons combine with molecular oxygen to form water, marking the terminal step of the ETC. Complex IV also pumps protons into the intermembrane space.
Throughout this process, protons accumulate in the intermembrane space, generating a proton motive force due to the difference in proton concentration and electrical charge across the inner mitochondrial membrane.
Oxidative Phosphorylation and ATP Synthesis
The proton motive force generated by the ETC drives the synthesis of ATP from ADP and inorganic phosphate, a process catalyzed by ATP synthase (Complex V). As protons flow back into the mitochondrial matrix via ATP synthase – down their electrochemical gradient – energy is released and used to synthesize ATP. This process of ATP production linked to electron transport is known as oxidative phosphorylation.
Electron Transport Chain and Thermogenesis
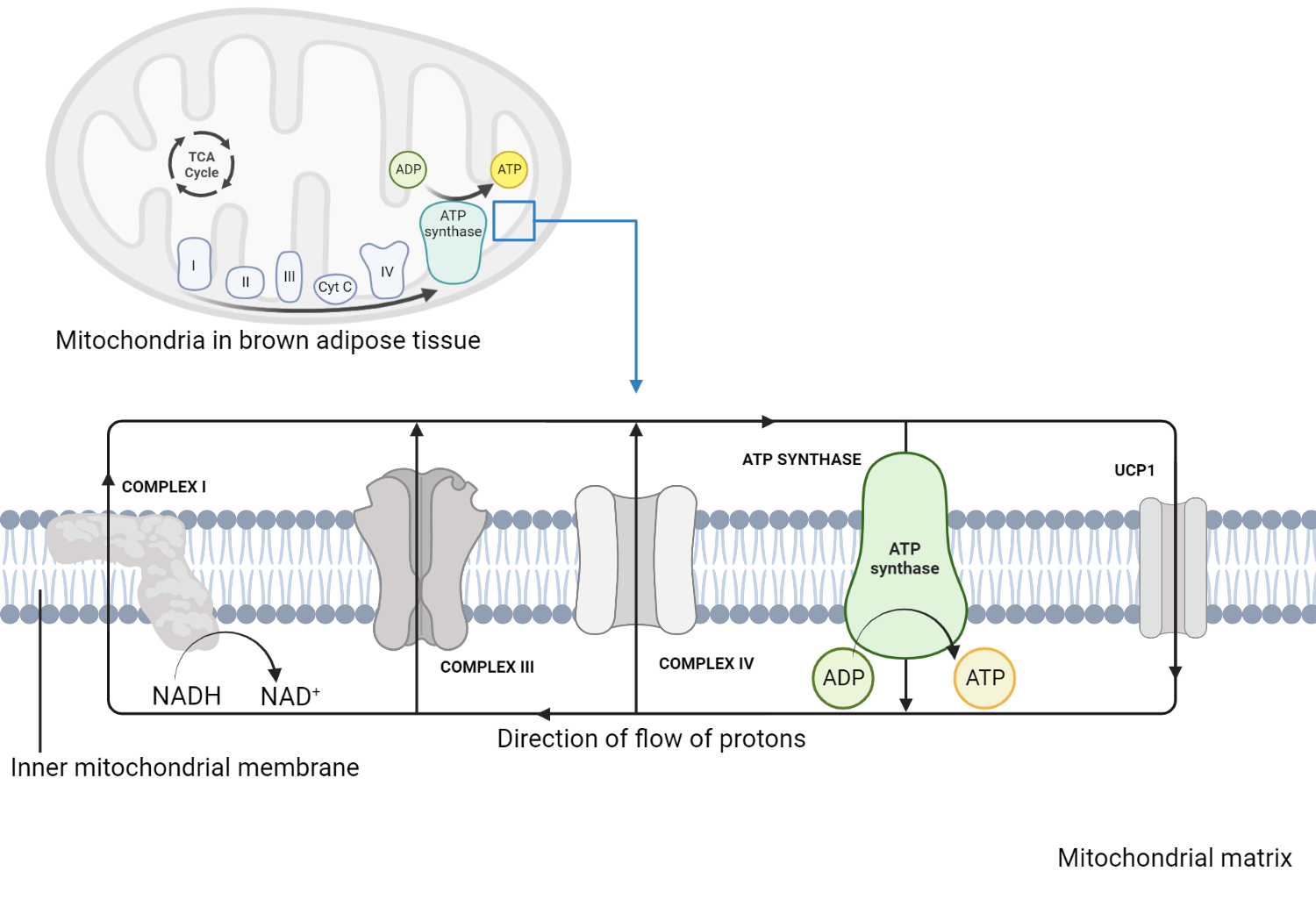
Uncoupling protein 1 (UCP1), also known as thermogenin, is a unique protein found in the mitochondria of brown adipose tissue (BAT). Its primary role is in non-shivering thermogenesis – the process of heat production that doesn’t involve muscle contractions.
Brown adipose tissue is different from the more common white adipose tissue (the body’s main fat storage form). The “brown” color comes from the high density of mitochondria and the rich blood supply in BAT. These mitochondria contain UCP1, which is essential for BAT’s function in thermogenesis.
When the body is exposed to cold or specific dietary inputs, UCP1 is activated, leading to heat production. Here’s how the process works:
- Under normal conditions, the electron transport chain in the mitochondria generates a proton gradient across the inner mitochondrial membrane by pumping protons from the mitochondrial matrix into the intermembrane space. This gradient drives ATP synthesis via ATP synthase, which allows protons back into the matrix and uses the energy released in the process to synthesize ATP.
- When UCP1 is activated, it provides an alternative pathway for protons to return to the matrix, bypassing ATP synthase. This “uncouples” the electron transport chain from ATP synthesis, hence the name “uncoupling protein.”
- Instead of driving ATP synthesis, the energy from the proton motive force is released as heat. This process contributes to body thermogenesis and helps maintain body temperature in cold environments.
UCP1 is an important therapeutic target in metabolic health research because its activation increases energy expenditure, potentially helping combat obesity and related metabolic disorders. Scientists are investigating ways to stimulate UCP1 activity or increase the amount of UCP1-containing brown fat in the body.
Conclusion
The electron transport chain is a crucial mechanism in bioenergetics. It enables efficient ATP production, serving as the principal source of cellular energy. A thorough understanding of the ETC is vital, given its importance in cellular metabolism and its involvement in numerous pathological conditions, including mitochondrial diseases, neurodegenerative disorders, and aging. Further research into the ETC may provide valuable insights into the development of therapies for these conditions.
Kindly Let Us Know If This Was helpful? Thank You!


