The juxtaglomerular apparatus is composed of the macula densa cells present in the distal convoluted tubule (DCT), juxtaglomerular cells (present in the entire glomerular vasculature but more prominent in the afferent arteriole) and the glomerular afferent arteriole(AT) [1].
See figure 1. Renin, an enzyme released from juxtaglomerular cells, initiates a cascade of reactions, which leads to the eventual formation of Angiotensin II(ATII)[2].
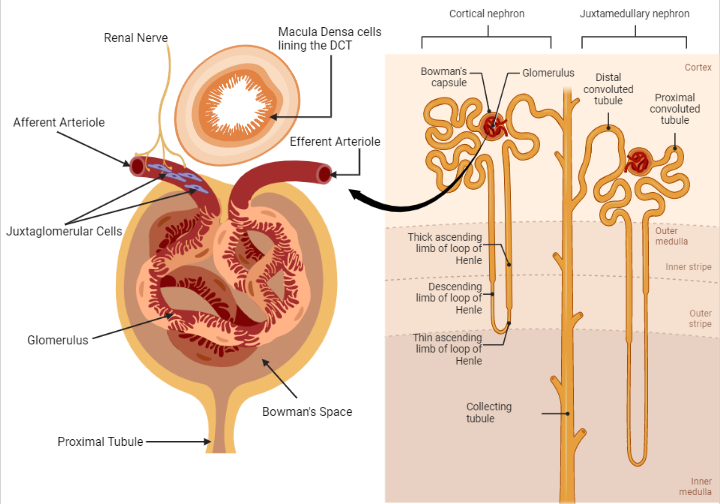
Fig. 1. The components of the juxtaglomerular apparatus. Triggers of renin secretion include a low sodium load presented to macula densa cells (located in the DCT), adrenergic stimulation from renal nerves innervating JGCs in the afferent arteriole and a reduced tensile stretch of the afferent arteriole of the glomerulus. Renin, the rate-limiting enzyme in the renin-angiotensin-aldosterone-system (RAAS) is critical in maintaining intravascular volume and blood pressure[2]. Renin release is also controlled by long and short negative feedback loops mediated by the effect of ATII on the collecting tubule and JGCs, respectively[4]. (Redrawn and modified from Alessandro et al. (2012) Role of the Renin-Angiotensin-Aldosterone System in the Pathogenesis of Atherosclerosis. Curr Pharm Des 18:981–1004)
The Mechanisms of Renin Release
- Change in sodium chloride load presented to the macula densa cells (a group of 15-20 cells in the distal convoluted tubule) influences the eventual release of renin from the juxtaglomerular cells (JGCs), through some signaling mediators. A high sodium load inhibits renin release from the juxtaglomerular cells, whereas a low sodium load does the opposite[3].
- A change in blood pressure at the afferent arteriole controls renin release from the JGCs. Low tension in the afferent arteriolar wall increases renin release, which ultimately enhances sodium conservation and maintenance of intravascular volume[3].
- Activation of beta-adrenergic sympathetic nerves terminating in the juxtaglomerular cells promotes renin release[3].
Aldosterone-mediated sodium and water conservation
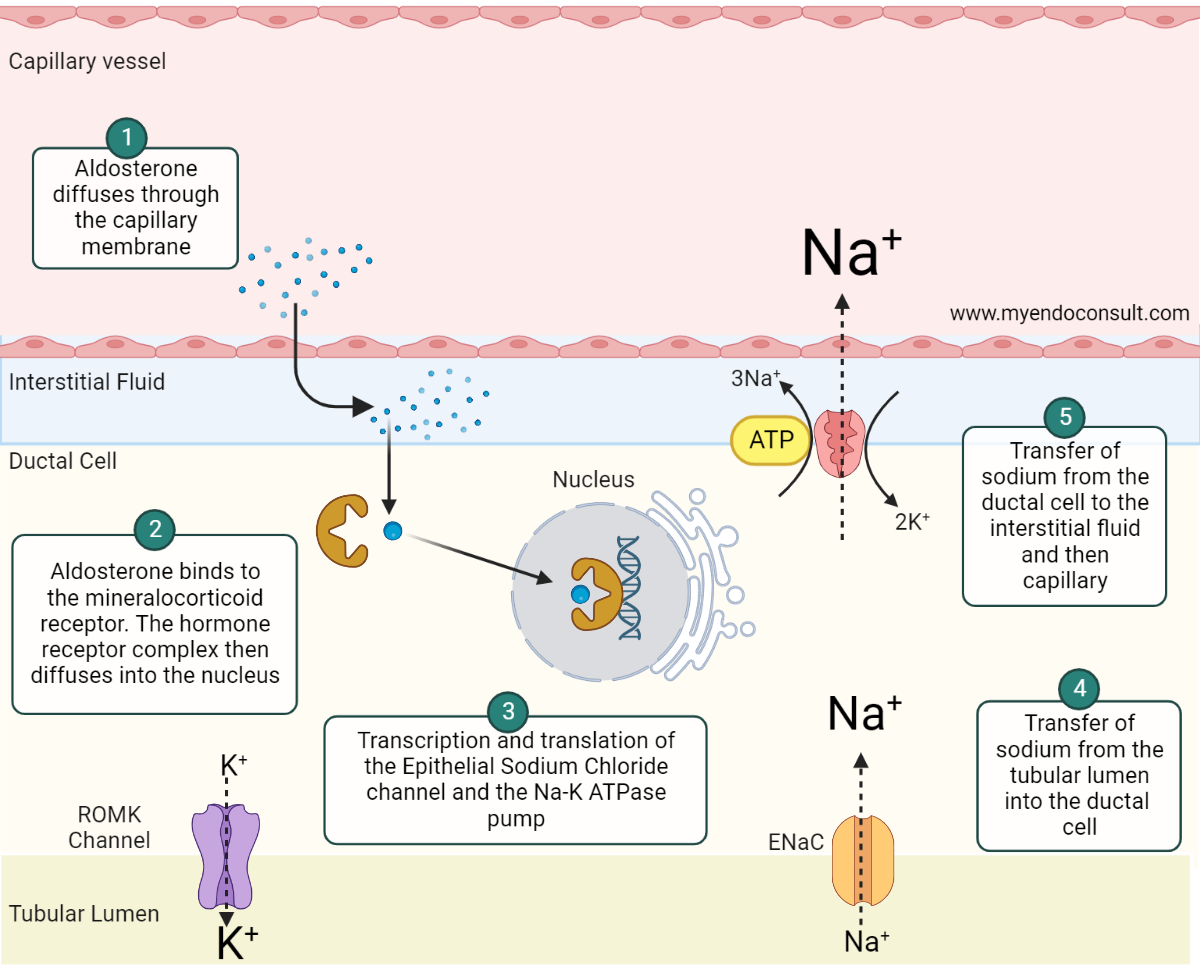
Fig. 2. Aldosterone mediated sodium and water conservation at the distal renal tubule. Aldosterone, a lipid-soluble steroid hormone, diffuses through the cell membrane of the ductal epithelial cell and binds to the cytosolic mineralocorticoid receptor (MCR)(step 1). The aldosterone-MCR complex is then translocated into the nucleus where it attaches to the hormone response element (HRE) (step 2) required for transcription and translation of the epithelial sodium chloride (ENaC) channel and sodium-potassium adenosine triphosphatase (Na-K+ ATPase) pump(step 3). ENaC and Na-K+ ATPase both play an active role in sodium reabsorption from the filtered renal sodium load present in the collecting duct and distal convoluted tubule. The net effect is the transfer of sodium from the apical to the basolateral side of the renal tubular cell(steps 4 and 5)[5, 6]. Aldosterone also promotes the expression of renal outer medullary potassium (ROMK) channels. The insertion of ROMK channels on the apical membrane facilitates potassium and hydrogen ion loss[7]. (Redrawn and modified from Byrd et al. (2018) Primary Aldosteronism. Circulation 138:823–835)
Clinical Pearl
Screening for hyperaldosteronism
Hypokalemia inhibits aldosterone production, as such, potassium levels should be maintained at a level ≥ 4mmol/L before screening for hyperaldosteronism with the Aldosterone to Renin Ratio (ARR)[5]. Medications that interfere with ARR testing, such as mineralocorticoid antagonists and angiotensin-converting enzyme (ACE) inhibitors, may not need to be held before initial hyperaldosteronism screening. Indeed, if renin is suppressed in the setting of mineralocorticoid antagonist or ACE inhibitor therapy, then the ARR is interpretable. It is essential to bear this caveat in mind since the discontinuation of antihypertensives comes with risks such as poorly controlled hypertension or, worse still, hypertensive crises[5].
References
- Barajas L (1979) Anatomy of the juxtaglomerular apparatus. Am J Physiol 237:F333-343
- Martini AG, Danser AHJ (2017) Juxtaglomerular Cell Phenotypic Plasticity. High Blood Press Cardiovasc Prev 24:231–242
- Peti-Peterdi J, Harris RC (2010) Macula Densa Sensing and Signaling Mechanisms of Renin Release. J Am Soc Nephrol 21:1093–1096
- Alessandro Durante, Giovanni Peretto, Alessandra Laricchia, Francesco Ancona, Marco Spartera, Antonio Mangieri and Domenico Cianflone (2012) Role of the Renin-Angiotensin-Aldosterone System in the Pathogenesis of Atherosclerosis. Current Pharmaceutical Design 18:981–1004
- Byrd James Brian, Turcu Adina F., Auchus Richard J. (2018) Primary Aldosteronism. Circulation 138:823–835
- Ong GSY, Young MJ (2017) Mineralocorticoid regulation of cell function: the role of rapid signaling and gene transcription pathways. Journal of Molecular Endocrinology 58:R33–R57
- Valinsky WC, Touyz RM, Shrier A (2018) Aldosterone, SGK1, and ion channels in the kidney. Clin Sci (Lond) 132:173–183
Kindly Let Us Know If This Was helpful? Thank You!



Excellent scientific presentation, illustration in the most possible simplified way. Thanks a ton