Glycogenesis is a metabolic process central to energy homeostasis in organisms, primarily responsible for the synthesis and storage of glycogen. Glycogen, a multi-branched polysaccharide of glucose, serves as a ready reservoir of glucose molecules, catering to the energy needs of the body during periods of fasting or intense physical activity. This article offers a detailed look at glycogenesis, its regulatory aspects, and its broader significance in human health and disease.
The Mechanism of Glycogenesis
Glycogenesis is a multi-step process that primarily takes place in the liver and skeletal muscles, the primary sites for glycogen storage. The process begins with glucose-6-phosphate (G6P), an intermediate molecule produced during glycolysis.
- Glucose-6-phosphate to Glucose-1-phosphate: The enzyme phosphoglucomutase catalyzes the rearrangement of G6P to glucose-1-phosphate (G1P).
- Glucose-1-phosphate to UDP-glucose: The enzyme UDP-glucose pyrophosphorylase catalyzes the reaction of G1P with UTP (uridine triphosphate), creating UDP-glucose and pyrophosphate.
- UDP-glucose to Glycogen: The enzyme glycogen synthase attaches UDP-glucose to the non-reducing ends of a pre-existing glycogen molecule. This forms α-1,4-glycosidic linkages and releases UDP.
- Branching of Glycogen: Every 8 to 12 glucose residues, the enzyme glycogen branching enzyme transfers a short chain of glucose units from the non-reducing ends to form a new branch with an α-1,6-glycosidic linkage.
The process continues until the glycogen molecule reaches its mature form, a highly branched structure with a large number of glucose units. The branching structure is vital for rapid glycogen synthesis and degradation because enzymes can work simultaneously on many branches.
Regulation of Glycogenesis
Glycogenesis is tightly regulated, ensuring that glucose storage adapts to the body’s energy needs. The most critical regulatory point is the enzyme glycogen synthase, controlled through complex mechanisms:
- Allosteric Regulation: Glycogen synthase is activated by glucose-6-phosphate, signifying the presence of excess glucose.
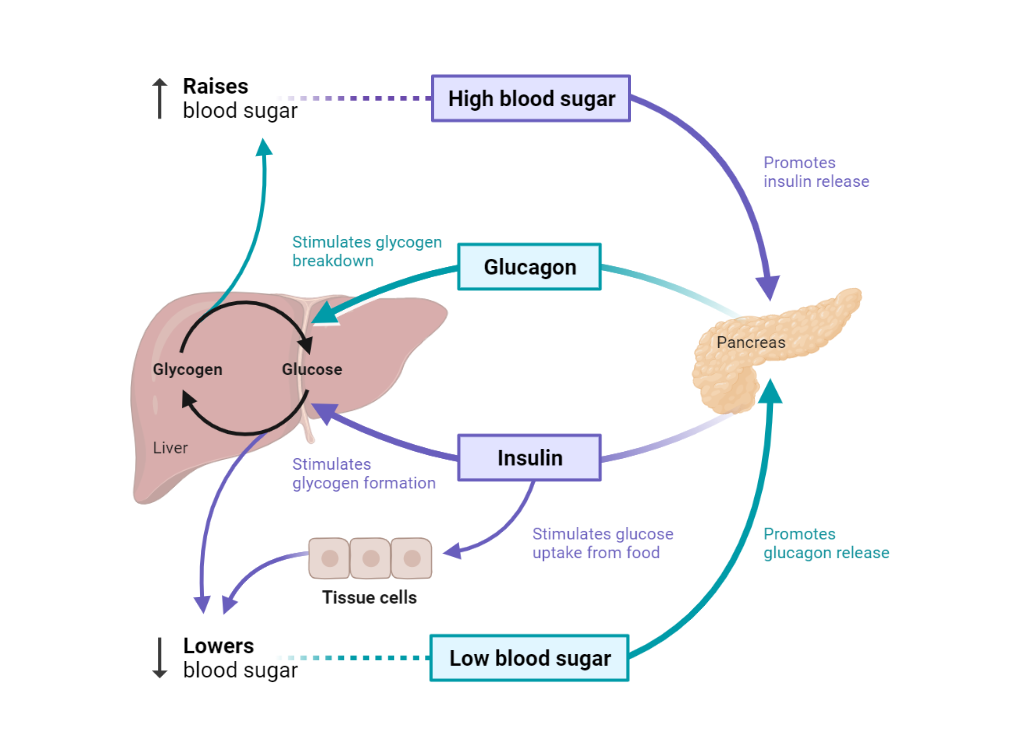
- Hormonal Regulation: Insulin promotes glycogenesis by activating protein phosphatase 1 (PP1), leading to the dephosphorylation and activation of glycogen synthase. In contrast, glucagon and adrenaline inhibit glycogenesis by activating glycogen synthase kinase 3 (GSK-3), resulting in the phosphorylation and inactivation of glycogen synthase.
- Covalent Modification: The phosphorylation state of glycogen synthase plays a significant role in its activity. Dephosphorylation activates the enzyme, while phosphorylation inhibits it.
The Role of Glycogenesis in Health and Disease
As a key player in glucose metabolism, glycogenesis plays a vital role in health and disease:
- Energy Homeostasis: Glycogenesis provides a way to store excess glucose as glycogen, a readily mobilizable energy source during periods of fasting, exercise, or stress.
- Disease States: Abnormalities in glycogenesis can lead to a group of conditions known as glycogen storage diseases (GSDs). These disorders are characterized by an inability to correctly synthesize or break down glycogen, leading to a variety of clinical manifestations depending on the specific enzyme deficiency involved.
- Potential Therapeutic Targets: Given its central role in glucose metabolism, understanding the regulation of glycogenesis has implications for the management of metabolic diseases such as diabetes. For instance, the action of insulin on glycogen synthase is of considerable interest, as insulin resistance is a primary defect in type 2 diabetes.
Glycogen Storage Diseases and Glycogenesis
Glycogen storage diseases (GSDs) represent a group of genetic disorders resulting from defects in the enzymes involved in glycogen synthesis or degradation. Depending on the specific enzyme affected, the location, structure, and amount of glycogen can be altered, leading to varying clinical manifestations.
For instance, GSD type I (von Gierke disease) is characterized by a deficiency in glucose-6-phosphatase, the enzyme that converts glucose-6-phosphate to glucose. This deficiency leads to severe hypoglycemia, lactic acidosis, and significant enlargement of the liver and kidneys due to excessive glycogen accumulation.
On the other hand, GSD type II (Pompe disease) involves a deficiency in the enzyme acid alpha-glucosidase, leading to glycogen accumulation in lysosomes primarily within muscle tissues, causing muscle weakness and respiratory difficulties.
Therapeutic Implications of Glycogenesis
Understanding the intricacies of glycogenesis can help in the development of novel therapeutic strategies for metabolic disorders, particularly type 2 diabetes. For instance, the promotion of glycogenesis in the liver could potentially lower blood glucose levels and improve glucose tolerance in individuals with type 2 diabetes. Similarly, enhancing glycogen storage capacity in skeletal muscles could improve exercise tolerance and insulin sensitivity.
Targeted therapies could involve drugs that activate glycogen synthase, inhibit glycogen breakdown, or enhance insulin signaling, thus promoting glycogen synthesis.
Conclusion
Glycogenesis, as a critical facet of glucose metabolism, plays a vital role in maintaining energy homeostasis within the body. Understanding this complex process and its regulatory mechanisms has profound implications for human health, particularly in the context of metabolic disorders such as diabetes and glycogen storage diseases. With ongoing research, there is hope that further insights into glycogenesis will open up novel avenues for therapeutic interventions, improving the lives of those affected by these conditions.
Kindly Let Us Know If This Was helpful? Thank You!


