A review of the standard inpatient hypoglycemia workup (also known as a 72hour fast protocol). Is the diagnostic algorithm for fasting hypoglycemia applicable to postprandial or reactive hypoglycemia?
The Structure of Insulin
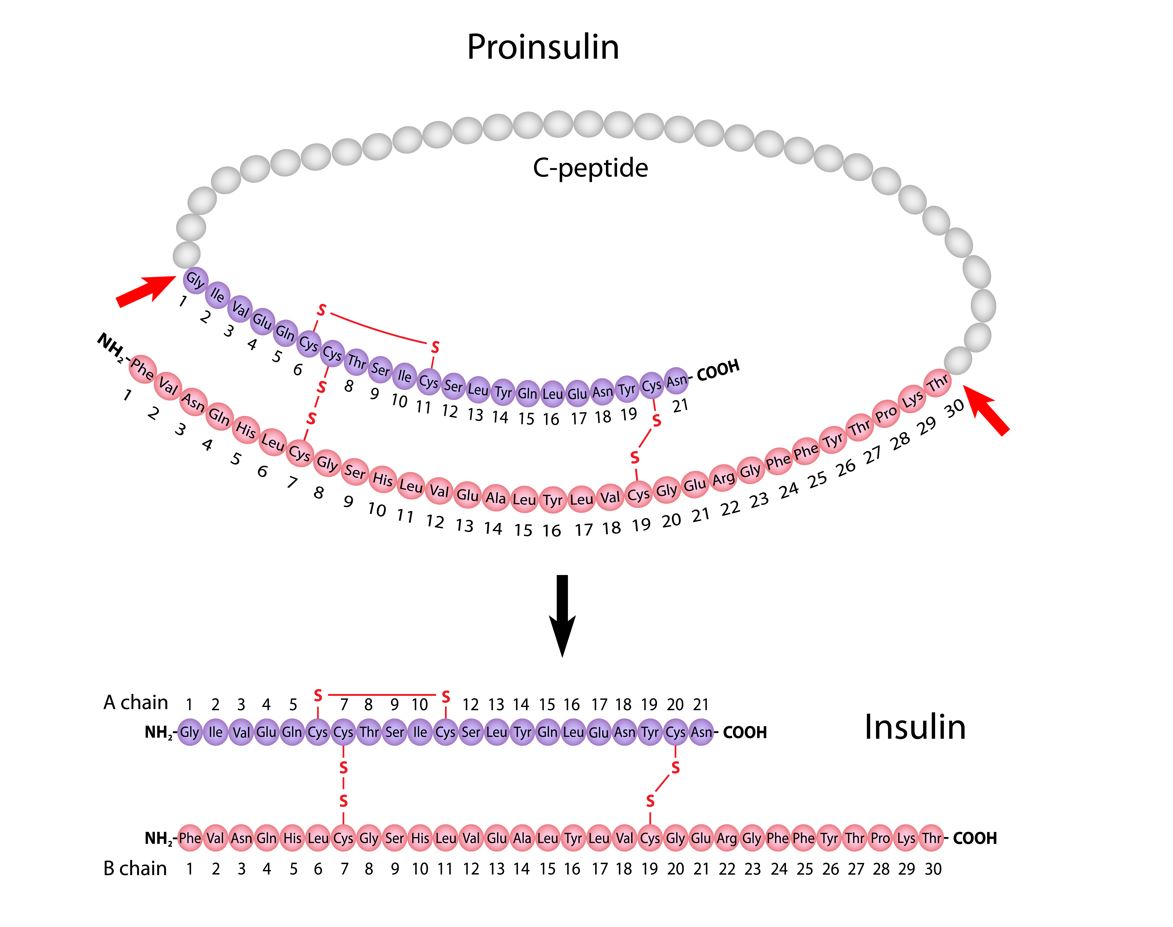
Proinsulin, a precursor to insulin, is comprised of alpha and beta chains that are connected by an amino acid sequence known as the “connecting peptide” or c-peptide. Subsequently, post translational modification of proinsulin results in the cleavage of c-peptide from the alpha and beta chains. The alpha and beta subunits/chains of insulin are joined by interchain disulfide bonds required to ensure insulin’s stability and biological activity.
Insulin Secretion
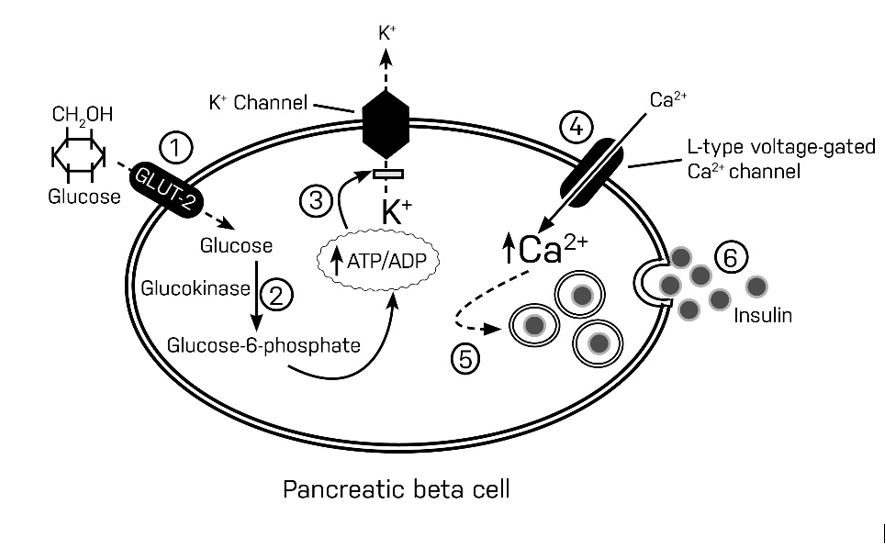
In the fasting (basal) state, the pancreatic cell membrane remains hyperpolarized, thus limiting insulin secretion. After a meal, glucose is actively transported into the cytoplasm of the pancreatic beta-cell by Glucose transporter 2 (GLUT-2)(step 1). Glucose goes through an initial phosphorylation step, which is facilitated by glucokinase (glycolysis)(step 2). Glycolysis generates a high concentration of intracytoplasmic ATP, which then inhibits ATP-sensitive potassium channels on the plasma membrane(step 3). Impaired potassium conductance leads to depolarization of the plasma membrane and subsequent activation of voltage-gated calcium channels. These activated calcium channels funnel calcium into the cytoplasm of the pancreatic beta-cell(step 4), where they mediate the release of preformed insulin from the secretory granules through a process of exocytosis(step 5 and 6).
Phases of insulin secretion
In the postprandial state, the first phase of insulin secretion is responsible for the suppression of hepatic glucose output. Insulin suppresses glycogenolysis by inhibiting glycogen phosphorylase (responsible for converting glycogen into glucose). Insulin also inhibits beta-oxidation (conversion of fatty acids into acetyl Coenzyme A) and proteolysis (degradation of proteins into constituent amino acids).
The second phase of insulin secretion regulates glucose uptake by insulin-dependent peripheral tissues such as muscle and fat.
What is Whipple’s Triad?
A triad of hypoglycemic symptoms biochemically confirmed hypoglycemia (plasma glucose < 70mg/dl) and reversal of hypoglycemic symptomatology after correction of hypoglycemia with an oral or intravenous glucose load.
Pathophysiology of Hypoglycemia
In response to hypoglycemia, there is a significant decline in the production of insulin by pancreatic beta cells. This is then followed by an increase in glucagon secretion by alpha cells of the pancreas. If hypoglycemia were to persist, additional counterregulatory hormones would be released – epinephrine release results in the typical hyperadrenergic symptoms of hypoglycemia. Finally, growth hormone and cortisol would be secreted as the final stress responses to hypoglycemia.
Symptoms, which prompt the behavioral defense of food ingestion, usually develop at a mean plasma glucose concentration of approximately 55 mg/dl (3.0 mmol/liter). At that and lower glucose levels, insulin secretion is suppressed virtually completely; plasma insulin levels are below 3 U/ml (18 pmol/liter), C-peptide levels are below 0.6 ng/ml (0.2 nmol/liter), and proinsulin levels are below 5.0 pmol/liter.
Evaluation of fasting hypoglycemia
In a non-diabetic patient, fasting hypoglycemia is extremely rare and, in most cases, warrants a thorough evaluation.
Hypoglycemia 72-hour fast protocol
– Discontinue dextrose-containing fluids briefly and monitor capillary glucose every 30 minutes. The patient should be instructed to call the nurse if he/she develops subjective hypoglycemic symptoms. If capillary glucose is less than 70 mg/dL, then venous glucose should be drawn immediately for confirmation in the lab.
– If true hypoglycemia is confirmed through venous glucose determination, a formal inpatient hypoglycemia workup would be required.
Initiate a formal fast.
Patient may receive normal or half-normal saline for hydration, but the intravenous MUST NOT contain dextrose. Liberal oral hydration (plain water) is permitted during the fast. A patient can also consume non-caloric caffeine-free beverages. All non-essential medications and supplements will need to be held during the fast.
Check capillary glucose every 1 hour during the fast.
Monitor patient for symptomatic hypoglycemia. If the patient has capillary glucose of 55 mg/dl or less with symptoms, promptly collect multiple blood samples during the event.
- Draw venous blood glucose level, insulin, proinsulin, beta-hydroxybutyrate, c-peptide, growth hormone, oral sulfonylurea screen, and cortisol levels.
- Administer IV glucagon 1mg stat as a treatment for hypoglycemia. Do not administer concentrated dextrose
- Recheck capillary glucose at 10 and 20 minutes after administering glucagon, and make a note of this in the medical record.
- Label all samples, including the date and time of the draw and HOLD.
Send a single sample (routine chemistry tube) for venous glucose estimation. If venous glucose is confirmed to be ( less than ) < 55 mg/dl, send all the additional blood samples and instruct the lab to run all additional lab tests as stipulated above.
Hypoglycemia Workup Algorithm
- Insulin or insulin-like action inhibits ketogenesis (this results in a low beta-hydroxybutyrate level).
- C-peptide, an essential component of insulin precursors, is present in the setting of endogenous insulin production (either due to a tumor or insulin secretagogue).
- Exogenous insulin contains only the alpha and beta chains; as such, c-peptide will remain undetectable among patients who inject insulin surreptitiously.
- In normal physiology, glycogen stores are expected to be depleted after a 72hour fast. As a result, glucose will fail to increase appreciably (that is, >25mg/dl) at the end of a fast, except in patients with persistent endogenous hyperinsulinemia.
In essence, if there is excess insulin (or insulin-like action), glycogen stores will never be completely depleted. These patients will therefore mount an appropriate increase in serum glucose (that is, >25mg/dl) after glucagon administration.
Furthermore, for normal persons with severe hypoglycemia at the end of a 72hour fast, insulin is expected to be appropriately low. Indeed, a relative decline in insulin levels will promote ketogenesis due to the action of multiple counterregulatory hormones.
| Glucose (mg/dl) | Insulin (microU/ml) | C-peptide (nmol/L) | Proinsulin (pmol/L) | Beta-Hydroxybutyrate (mmol/L) | Glucose increase after glucagon (mg/dl) | Diagnosis |
| <55 | <3 | <0.2 | <5 | >2.7 | <25 | Normal |
| <55 | >>3 | <0.2 | <5 | <2.7 or normal | >25 | Exogenous Insulin |
| <55 | >3 or normal | >0.2 or normal | >5 or normal | <2.7 or normal | >25 | Insulinoma or NIPHS |
| <55 | >3 or normal | >0.2 or normal | >5 or normal | <2.7 or normal | >25 | Oral Hypoglycemic Agent |
| <55 | >>3 | >>0.2 | >>5 or normal | <2.7 or normal | >25 | Autoimmune : Agonist Antibodies to insulin |
| <55 | <3 | <0.2 | <5 | <2.7 or normal | >25 | IGF-II mediated |
| <55 | >3 | <0.2 | <5 | <2.7 or normal | >25 | Autoimmune : Insulin receptor antibodies |
Practice Pearl
Interpretation of the above tests in postprandial hypoglycemia or reactive hypoglycemia is fraught with a significant diagnostic challenge.
C-peptide has a half-life of approximately 30 minutes, meaning c-peptide may remain detectable even if insulin is appropriately suppressed during postprandial hypoglycemia.
References
- Philip E. Cryer, Lloyd Axelrod, Ashley B. Grossman, Simon R. Heller, Victor M. Montori, Elizabeth R. Seaquist, F. John Service, Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 94, Issue 3, 1 March 2009, Pages 709–728, https://doi.org/10.1210/jc.2008-1410
- Yevgeniya Dynkevich, Kristina I. Rother, Ian Whitford, Sana Qureshi, Sneha Galiveeti, Alessandra L. Szulc, Ann Danoff, Tracy L. Breen, Nargess Kaviani, Michael H. Shanik, Derek LeRoith, Riccardo Vigneri, Christian A. Koch, Jesse Roth, Tumors, IGF-2, and Hypoglycemia: Insights From the Clinic, the Laboratory, and the Historical Archive, Endocrine Reviews, Volume 34, Issue 6, 1 December 2013, Pages 798–826
Kindly Let Us Know If This Was helpful? Thank You!


