Kussmaul breathing is a type of deep, rapid breathing that occurs when the body is trying to get rid of excess carbon dioxide.
This eponymous term is attributable to the German doctor who first described it, Adolf Kussmaul (1822-1902). The clinical features and pathophysiologic mechanisms underlying this sign will be reviewed.
What is Kussmaul breathing
Kussmaul aptly described these findings in his original description as follows, “A peculiar type of dyspnea…there is not the least suggestion…that the passage of air to or from the lung has to combat obstruction…everything is indicative of air hunger…even when the patients lies unconscious in deep coma” [1]
History of this physical sign
Kussmaul breathing is a labored breathing pattern that is characterized by a deep and rapid hyperventilation pattern of breathing. It was first described by German doctor Adolf Kussmaul in 1874 [2].
Kussmaul was studying a group of diabetic patients with advanced diabetes mellitus (insulin deficiency) in severe acidosis and observed that they had a peculiar form of breathing. He noted that their breathing pattern was deep and rapid and occurred prior to death [3].
Clinical Diagnosis of Kussmaul breathing
Kussmaul breathing (acidotic breathing) is characterized by deep, rapid breaths that are usually accompanied by a noticeable change in the person’s respiration rate. The breaths may be so deep that they cause the person’s chest to rise and fall noticeably on physical examination.
https://youtu.be/ebgxcj3eJLA
Characteristic deep and rapid breathing pattern in a patient with ketoacidosis. Video Credit : Youtube @Jess Suchard
In subjects with significant metabolic acidosis as might occur in diabetic ketoacidosis, they develop a terminal, deep, and rapid respiratory pattern called Kussmaul’s respiration/breathing. This respiratory pattern heralds an impending state of respiratory failure which may invariably require assisted ventilation[4].
It is worthy to note that a characteristic fruity odor due to the presence of acetone (somewhat similar to the smell of nail polish remover) can be appreciated during the bedside physical examination[5].
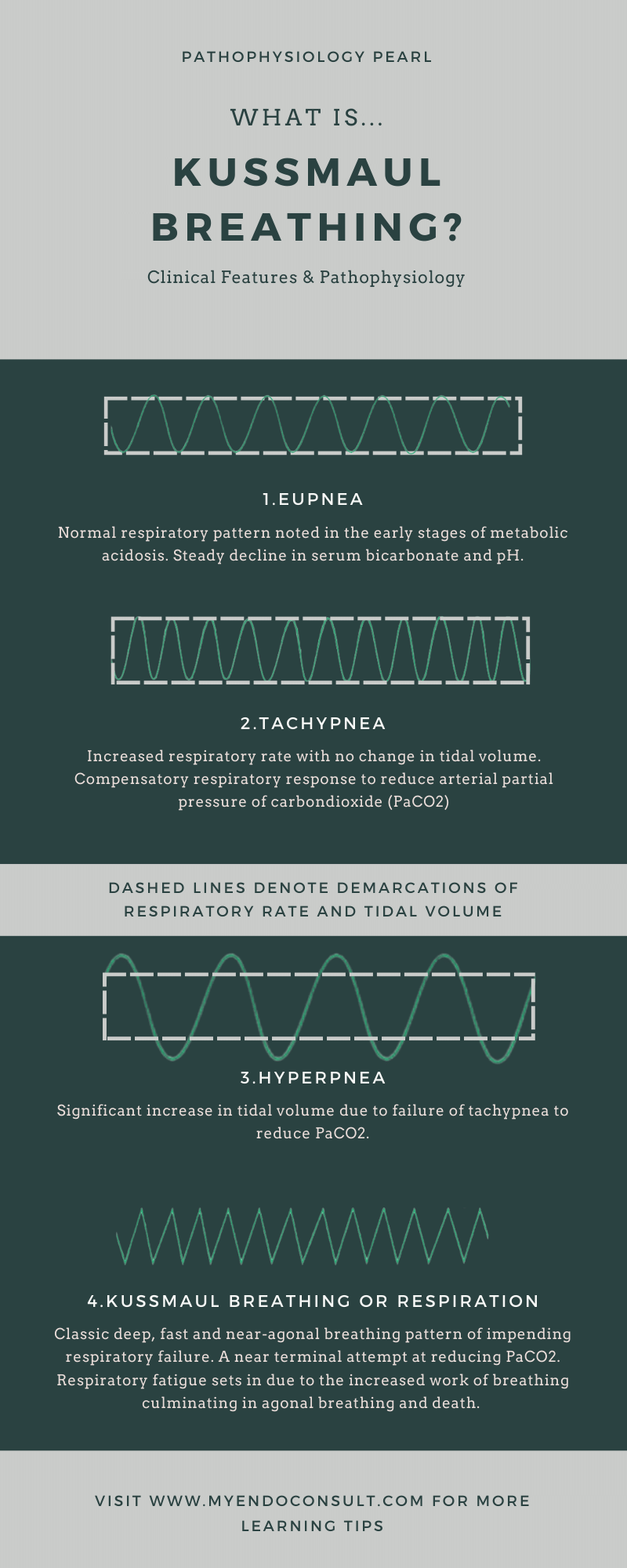
This pattern of acidotic breathing is the final stage of the sequence of respiratory patterns that typically begins with tachypnea (increased respiratory rate), followed by hyperpnea (increased tidal volume) which then transitions to the classic deep, fast, and agonal pattern of Kussmaul’s respiration. The clinical findings of the sequence of events leading to terminal Kussmaul breathing are shown in the figure below.
Stages of Kussmaul Respiration
- There is an initial phase of normal respiratory pattern even in the setting of a steady decline in serum bicarbonate and pH (Eupnea)
- This is followed by increased respiratory rate with no change in tidal volume (tachypnea) – a compensatory respiratory response which aims to reduce arterial partial pressure of carbondioxide (PaCO2).
- The next phase is characterized by an increase in tidal volume due to failure of tachypnea to reduce PaCO2.
- The final phase is characteristic by a classic deep, fast and near agonal breathing pattern of impending respirtaory failure. This is a near terminal attempt at reducing PaCO2. Finally, respiratory fatigue sets in due to the increased work of breathing. If the patient is not provided with assisted ventilation and correction of underlying acidosis, this will progress to agonal breathing and unfortunately, death. An astute clinician should be able to recognize the urgency of life saving intervention in this terminal respiratory pattern.
Causes of Kussmaul breathing
This type of breathing is often seen in people with conditions that affect the lungs’ ability to exchange oxygen and carbon dioxide. It has classically been described in people with certain types of acidosis, such as diabetic ketoacidosis.
Kussmaul breathing develops in any cause of metabolic acidosis (usually of rapid onset) [6]. A mnemonic for remembering the causes of metabolic acidosis can be found here.
- Diabetic ketoacidosis (elevated blood glucose and ketone bodies)
- Acute renal failure
- Chronic renal failure
- Alcoholic ketoacidosis
- Lactic acidosis (both L-lactic and D-lactic acidosis)
- Starvation ketoacidosis
- Salicylate poisoning
- Alcohol intoxication (Ethylene glycol, methanol, propylene glycol or diethylene glycol)
- Toluene poisoning
- Pyroglutamic acidosis
Mechanism of metabolic acidosis
Metabolic acidosis occurs due to either a loss of serum bicarbonate or an accumulation of nonvolatile acids (that cannot be eliminated through respiratory responses). Renal acidification processes also play a pivotal role in regulating hydrogen ions (required for buffering serum bicarbonate).
Pathophysiology of Kussmaul breathing
Metabolic acidosis triggers a compensatory increase in the respiratory rate in order to reduce dissolved carbon dioxide (CO2) and thus maintain serum pH. An initial phase of tachypnea progresses through hyperpnea (increased tidal volume) and culminates into the pre-terminal respiratory pattern of Kussmaul respiration[4].
It is worthy to note that respiratory responses to metabolic acidosis initiate a cascade of events that may result in terminal agonal breathing and death if left unchecked. In effect, the compensatory mechanism against too much acid in the blood leads to a life-threatening state.
A reduction in serum bicarbonate and pH for patients in metabolic acidosis initiates compensatory hyperventilation in a bid to reduce serum carbon dioxide thus preventing further decline in serum pH. For every 1 mEq/L decline in serum bicarbonate (metabolic acidosis), respiratory compensatory mechanisms lead to a 1.2 mmHg decline in arterial CO2 [7].
In normal physiology, respiratory compensation occurs within half an hour and is usually complete within 24 hours of the onset of metabolic acidosis. However, in patients who develop rapid onset metabolic acidosis as might okay in diabetic ketoacidosis, respiratory responses are unable to keep up with the rate of decline in serum bicarbonate (sometimes in excess of 4 mEq/L over six hours).
The goal of respiratory responses is to blow off enough CO2 to curtail a further decline in serum pH. Although initial tachypnea (fast breathing) and hyperpnea (increased tidal volume) aim to eliminate carbon dioxide at an accelerated rate, there is a limit to the nurse prior to systems capacity to achieve this goal.
Indeed, for patients with serum bicarbonate concentration below 6 mEq/L, arterial CO2 cannot fall lower than 8–10 mmHg. This is due to the contribution of dead space ventilation.
An additional limiting factor is the fact that respiratory muscles can only compensate for a limited time and eventually, patients develop fatigue due to increased work of breathing. This rapidly accelerates acidosis and leads to death if no intervention occurs[4].
Patients with Kussmaul respiration may be close to terminal agonal breathing; as such, the need for mechanical ventilation should be under consideration in this patient subset. Low tidal volume ventilation reduces the risk of acute respiratory distress syndrome in diabetic patients with severe acidosis.
Also, Kussmaul breathing, a compensatory response to metabolic acidosis increases intra-alveolar pressures (overinflation of the air sacs) and may predispose patients to alveolar rupture and pneumomediastinum (Hamman’s syndrome)[8].
Treatment of Kussmaul Breathing
Treatment of the underlying cause of Kussmaul’s breathing results in the gradual resolution of metabolic acidosis. For patients with diabetic ketoacidosis, this involves aggressive intravenous fluid administration and the institution of continuous IV regular insulin.
Diabetic ketoacidosis can be precipitated by an infection, myocardial infarction, or medication non-adherence. Supplemental oxygen helps in reducing the work of breathing.
Prognosis of Kussmaul Breathing
Poor recognition of this near terminal respiration progresses to respiratory muscle fatigue, general exhaustion accompanied by rapid accumulation of carbon dioxide (a mixed acidotic state) and death.
What is the difference between Kussmaul and Cheyne Stokes respiration?
Both Kussmaul and Cheyne Stokes’ respiration resemble fast breathing in the setting of a high arterial CO2. Kussmaul’s breathing however does not alternate from fast breathing to slow breathing as Cheyne Stokes does.
References
1. Bast TH. The Life & Time of Adolf Kussmaul. PB Hoeber, Incorporated; 1926.
2. Kussmaul A. Zur Lehre vom Diabetes mellitus. Über eine eigenthümliche Todesart bei Diabetischen, über Acetonämie, Glycerin-Behandlung des Diabetes und Einspritzungen von Diastase in’s Blut bei dieser Krankheit. Deutsches Archiv klinische Medicin, Leipzig. 1874; 14: 1–46.[Thomas CC. English translation in Ralph Hermon Major (1884–1970), classic descriptions of disease. Springfield; 1932. 1939. 1945]. Emmett M. Anion-gap interpretation: the old and the new. Nat Clin Pr Nephrol. 2006;2(1):4-5.
3. Johnson SK, Naidu RK, Ostopowicz RC, et al. Adolf Kussmaul: distinguished clinician and medical pioneer. Clin Med Res. 2009;7(3):107-112. doi:10.3121/cmr.2009.850
4. Gallo de Moraes A, Surani S. Effects of diabetic ketoacidosis in the respiratory system. World J Diabetes. 2019;10(1):16-22. doi:10.4239/wjd.v10.i1.16
5. Seth P, Kaur H, Kaur M. Clinical Profile of Diabetic Ketoacidosis: A Prospective Study in a Tertiary Care Hospital. J Clin Diagn Res JCDR. 2015;9(6):OC01-OC04. doi:10.7860/JCDR/2015/8586.5995
6. Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6(5):274-285. doi:10.1038/nrneph.2010.33
7. Albert MS, Dell RB, Winters RW. Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann Intern Med. 1967;66(2):312-322. doi:10.7326/0003-4819-66-2-312
8. Pain AR, Pomroy J, Benjamin A. Hamman’s syndrome in diabetic ketoacidosis. Endocrinol Diabetes Metab Case Rep. 2017;2017. doi:10.1530/EDM-17-0135
Kindly Let Us Know If This Was helpful? Thank You!


