Gonadal Structures and Reproductive Mechanisms
The gonads, consisting of the ovaries in women and the testes in males, are critical for two primary functions: gametogenesis, the production of germ cells such as ova or sperm, and the secretion of certain steroid sex hormones that facilitate the normal growth and functionality of reproductive organs, along with the distinction of secondary sexual characteristics. These hormones also play a central role during gestation, parturition, and lactation.
The gonads secrete three varieties of sex hormones: androgens (example: testosterone), which are male sex hormones, estrogens (example: 17β—estradiol), the female sex hormones, and progestogens (example: Progesterone), another category of female sex hormones. Androgens and oestrogens are responsible for inducing masculine or feminine effects within the body, respectively, while progestogens primarily influence the uterus, preparing it for gestation and subsequently supporting it.
Male gonads, the testes, participate in a constant production of sperm and testosterone from puberty, which typically begins around 13 years of age, until senescence. On the contrary, in the adult female, usually a single ovum is generated approximately every 28 days, accompanied by cyclical estrogen and progesterone secretion (the menstrual cycle). This cycle continues until menopause, which marks the end of the ovarian cycle.
The operation of the gonads and sexual function are modulated primarily by the hypothalamus and anterior pituitary, influenced by the higher centers of the brain, such as the cortex. The onset of puberty is believed to be precipitated by maturation and subsequent “release from inhibition’ of the hypothalamic gonadotrophin-releasing hormone (GnRH) neurons within the arcuate nucleus.
This process leads to the establishment of pulsatile GnRH release, which occurs every 60 to 90 minutes, and subsequently drives an increase in pulsatile gonadotrophin secretion from the pituitary. Precocious puberty in children could potentially result from premature activation of this hypothalamic-pituitary system caused by conditions such as brain tumors or hydrocephalus.
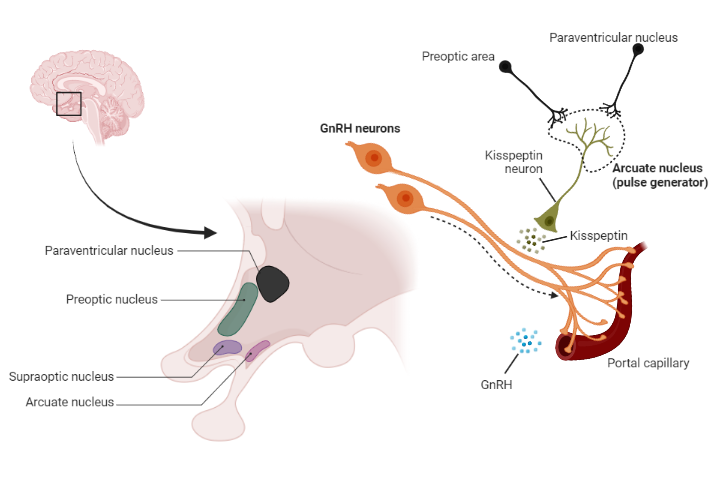
Kisspeptin, also known as metastin, has emerged as a key regulator of GnRH neurons in the HPA. Kisspeptin is a peptide hormone encoded by the KISS1 gene. It was discovered for its role in the initiation of puberty. Kisspeptin is primarily expressed in the hypothalamus, particularly in the arcuate nucleus and anteroventral periventricular nucleus.
Kisspeptin plays a dual role in the regulation of the HPA through positive and negative feedback loops. During puberty, kisspeptin stimulates the release of GnRH, which initiates the secretion of FSH and LH, leading to the development of secondary sexual characteristics. This positive feedback loop is crucial for the onset of puberty.
The Male Reproductive System: Structural and Histological Overview
The testes, similar to the female ovaries, perform both endocrine and exocrine functions. Each testes, roughly weighing 20 grams and located in the scrotum, are predominantly composed of a multitude of coiled seminiferous tubules encased in a fibrous capsule. The interior walls of these tubules contain a significant number of spermatogenic cells (spermatogonia, spermatocytes, and spermatids) which undergo various stages and divisions to generate spermatozoa.
Additionally, the tubular epithelium houses larger Sertoli cells that stretch from the basal membrane to the tubular lumen. These cells are involved in the nourishment and support of sperm cells during their maturation process. The existence of tight junctions between adjacent Sertoli cells formulates a ‘blood-testes barrier.’ This barrier separates the basal and adluminal compartments of the tubule, producing a specialized environment for more developed germ cells and potentially safeguarding them from harmful substances present in the general circulation. Fully matured sperm are liberated from the apex of Sertoli cells into the seminiferous tubules at a rate of approximately 100–200 ×106 per day.
Positioned between the seminiferous tubules, along with blood vessels, lymphatics, and nerves, are clusters of interstitial Leydig cells. These cells synthesize and secrete testosterone into the bloodstream. Furthermore, Sertoli cells have the ability to convert androgens to estrogens, which might serve a localized paracrine function in Leydig cells to limit testosterone production. However, the majority (about 70%) of the estrogen present in adult males’ circulation is nontesticular, derived from circulating testosterone and androstenedione through the enzymatic activity of aromatase in peripheral tissues, chiefly adipose tissue and the liver. The rate of this extraglandular estrogen production tends to escalate with age.
Demystifying the Mastery of Spermatogenesis and Hormone Regulation
The testes serve as maestro, conducting an orchestra of hormones, managing the symphony of spermatogenesis, the process of sperm creation. A key player in this elaborate biological process is the hypothalamic Gonadotropin-Releasing Hormone (GnRH), which stirs the anterior pituitary gonadotrophins, LH (Luteinizing Hormone) and FSH (Follicle Stimulating Hormone), into action. Together, these hormones hold the reins of testicular function, orchestrating the complex dance of sperm production in the seminiferous tubules.
Right at the onset of this intricate performance, FSH steps forward, influencing Sertoli cells – the ‘nursemaids’ of developing sperm cells, to kickstart spermatogenesis. As the show progresses, LH takes over the leading role, guiding Leydig cells to produce testosterone. This testosterone then spreads its influence on neighboring Sertoli cells, a pivotal interaction that ensures the smooth continuation of spermatogenesis. Moreover, testosterone oversees the final act of spermatogenesis, guiding the maturation of spermatids into fully mature spermatozoa.
Intriguingly, FSH and testosterone work in tandem to stimulate Sertoli cells to produce androgen-binding protein (ABP), a vital player that locks testosterone into the tubular lumen fluid. This process helps maintain the required high intratubular concentration required for this critical hormone, an essential condition for normal spermatogenesis. The mechanism of FSH’s actions is mediated via specific FSH receptors located primarily on Sertoli cells. These receptors are linked to cAMP production, a vital cellular messaging molecule. In addition, both LH and FSH exert their influence on the Leydig and Sertoli cells, respectively, promoting growth and development, a key aspect of healthy testicular function.
Switching gears to the control of testosterone release, this falls primarily into the domain of LH. The hormone acts on Leydig cells, a process mediated by LH receptors on the cell surface linked to intracellular cAMP production. Interestingly, testosterone keeps an eye on LH release through a combination of direct and indirect negative feedback mechanisms, acting on the anterior pituitary and hypothalamus, respectively. However, it usually hampers FSH release. Instead, this job is believed to be managed by inhibin, a peptide hormone secreted by Sertoli cells under the influence of FSH.
This inhibin is quite a complex character – it is a dimeric 32 kDa glycoprotein made up of two distinct subunits, α and β, with two forms of the β chain, A and B. What is even more fascinating is that there is another structurally similar peptide, activin, formed from two inhibin β chains. This molecule, secreted primarily by testicular Leydig cells in males, plays a sort of contrarian role, showing effects counter to inhibin on pituitary FSH release and gonadal steroidogenesis. Both inhibin and activin are not only involved in the reproductive system; they could also orchestrate early cellular differentiation and development, while producing notable local effects on adjacent Leydig and Sertoli cells. Additionally, there is a prospect of inhibin being used as a male contraceptive agent, opening fascinating avenues for future research.
Another compelling molecule in this mix is follistatin, a monomeric glycoprotein also present in ovarian follicular fluid. Although it is not structurally related to either inhibin or activin, it demonstrates an inhibitory effect on pituitary FSH release, similar to, but less potent than, inhibin. An intriguing characteristic of follistatin is its affinity for activin. It binds to circulating activin with high affinity, effectively neutralizing it and curtailing its biological impacts across a variety of tissues.
This discovery paints a dynamic picture of the diverse roles that follistatin could play within our bodies. However, the role it plays in human reproduction is interestingly still somewhat murky. Follistatin remains a molecule of interest, and further studies into its function and influence on human reproduction could illuminate its role more clearly.
Furthermore, a peptide similar to inhibin has been identified in the adrenal glands. This discovery hints at the possibility that inhibin-like peptides could be key players in the proliferation and differentiation. These inhibin-like peptides could extend their regulatory influence beyond the confines of the reproductive system, perhaps serving crucial roles in other physiological systems as well.

Unraveling the Role of Testosterone A Hormonal Heavyweight
Testosterone, the dominant steroid hormone secreted by the testes, is vital in the symphony of human endocrinology. Testosterone plays a pivotal role in our biological processes, primarily derived from low-density lipoprotein (LDL) cholesterol and subsequently converted to pregnenolone. Although its most significant production site is the testes, it also appears in cameo appearances in the adrenal cortex of both sexes and in the ovaries of females.
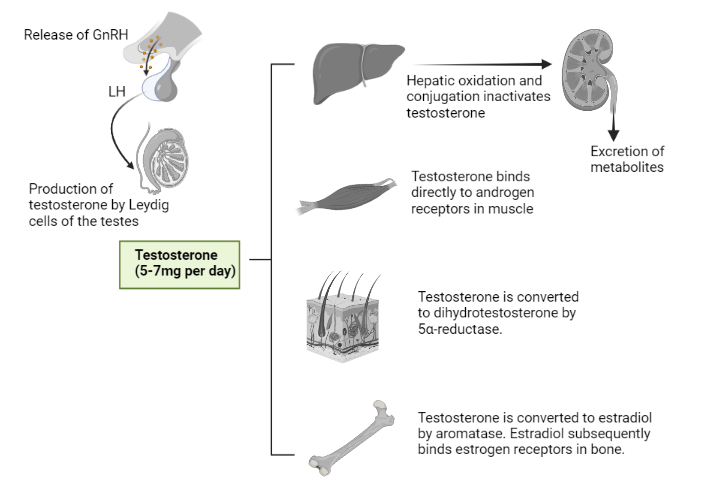
It should be noted that nearly 98% of the testosterone found in plasma is bound to proteins. A large portion (around 65%) is related to sex hormone binding globulin (SHBG), a product of liver synthesis, and the remaining testosterone attached to albumin and other proteins. Unbound (or biologically active) testosterone has a rather short lifespan in plasma, approximately 10 to 20 minutes, before it is metabolized primarily into inert 17-ketosteroids and inert polar metabolites. These products are rapidly excreted in the urine. But don’t let the short half-life fool you. Albumin-bound testosterone can easily dissociate, making ‘bioavailable’ testosterone a combination of both unbound and albumin-bound hormones.
Testosterone is known for its pivotal role in male reproductive development. Not only stimulates the growth of the entire male genital tract, but also boasts potent protein anabolic properties, leading to increased muscle mass and nitrogen retention.
The transformative power of testosterone can be observed at various stages of life:
- In utero, testosterone produced by embryonic testes during a crucial fetal period (8-12 weeks) is responsible for the differentiation of the male internal and external genitalia and the descent of the testes into the scrotum. The fetal testes also secrete a substance called Müllerian inhibitory substance, which inhibits the development of female genital ducts in male fetuses.
- Throughout puberty, testosterone is at the helm of male sex organ growth. It contributes to the pubertal ‘growth spurt’, prompts the fusion of the bone ends or epiphyses of long bones, leads to the deepening of the voice, stimulates hair growth in various areas, and amplifies muscularity and strength. It even influences the activity of the sebaceous glands, which can sometimes lead to acne.
- In adulthood, testosterone helps to maintain masculinity, libido (sexual drive), and sexual potency. It collaborates with FSH in the regulation of spermatogenesis in the testes and plays an integral part in feedback mechanisms in the hypothalamus and pituitary. It is also implicated in hairline recession and baldness in genetically predisposed individuals. An interesting fact is that bioavailable testosterone slowly declines with age in healthy men, with a drop of about 1% per year between the ages of 40 and 70, but without a dramatic decrease like that observed in the female menopause (a period of loss of ovarian function).
Deciphering Testosterone’s Modus Operandi
Testosterone, like other steroid hormones, performs its functions through an intricate interaction with an intracellular androgen receptor protein (AR) that shows a high affinity for the hormone. This coupling enables the testosterone-AR complex to bind to specific androgen-responsive elements (AREs) in nuclear DNA, influencing the transcription of particular genes, thus modulating the synthesis of specific proteins within the cell cytoplasm.
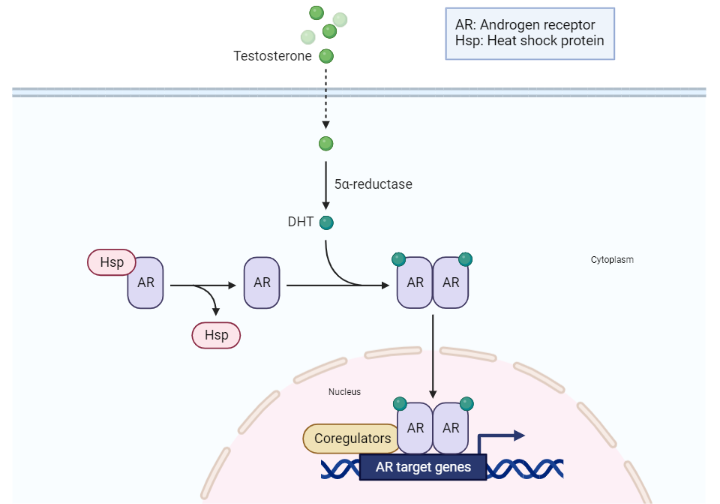
In certain target tissues, such as the skin, prostate gland, and seminal vesicles, testosterone undergoes conversion to a more potent derivative, 5α-dihydrotestosterone (DHT). This transformation is orchestrated by the nuclear enzyme 5-α-reductase. Remarkably, DHT not only interacts with the same intracellular receptor as testosterone, but exhibits a two-fold higher binding affinity and a slower dissociation rate. This results in a pronounced androgenic activity at these sites. Therefore, testosterone also moonlights as a prohormone, instigating intracellular DHT production within specific androgen-responsive tissues.
It should be noted that differentiation of the male external genitalia and prostate gland during fetal development necessitates the action of DHT, not testosterone. On the contrary, testosterone takes the reins in overseeing the development of the Wolffian ducts into male internal genitalia. Additionally, DHT plays a pivotal role in driving the growth of the prostate and penis during puberty and instigating the development of facial hair, acne, and possibly scalp recession in later life.
In women, androstenedione serves as the primary precursor for DHT production in the skin. Unlike testosterone, DHT is a non-aromatizable androgen, which means it is immune to aromatase conversion into estrogens.
A peek into the developmental timeline of a male embryo reveals another actor on the hormonal stage. Immature Sertoli cells secrete an inhibin-like peptide known as Müllerian inhibitory substance (MIS). This substance instigates regression of the Müllerian duct system, which would otherwise develop into fallopian tubes and a uterus. MIS, a member of the transforming growth factor-beta (TGF-β) superfamily of peptides, plays an important role in regulating cell growth and differentiation. It needs enzymatic processing by a protease to become biologically active.
Interestingly, MIS is present in the testes postnatally for several years, declining to low levels after early puberty. In women, MIS production kicks off at puberty in the ovaries and continues throughout reproductive life, which implies a potential role in controlling oocyte maturation. Monitoring serum MIS levels can be a valuable diagnostic tool for patients with various congenital intersex and gonadal abnormalities.
Exploring Clinical Disorders Associated with Testosterone
Reduced testosterone secretion or functionality in males, referred to as hypogonadism, can arise due to a variety of abnormalities:
- Primary hypogonadism is characterized by the absence of functional testes at birth, often resulting from chromosomal abnormalities.
- Secondary hypogonadism, or hypopituitarism, can arise from the underdevelopment of the testes due to insufficient secretion of pituitary gonadotrophins.
- Cryptorchidism refers to the condition in which the fetal testes do not descend from the abdomen into the scrotum.
- Testicular loss or destruction by disease, before or after puberty, can also lead to hypogonadism.
In the event of loss of testicular function before puberty, one can anticipate a failure in the normal development of the sexual organs and secondary sexual characteristics can be predicted. In adults, this would typically manifest itself as a decrease in libido, some regression of masculine characteristics, and infertility.
Androgen replacement therapy is typically the course of treatment for hypogonadism, the specifics of which are determined by the root cause and the age of onset. Cryptorchidism can be addressed (before puberty) by intramuscular administration of gonadotrophin or by surgical intervention.
The congenital absence of enzymes pivotal to testosterone action (like 5-α-reductase deficiency, leading to male pseudohermaphroditism) or molecular defects at the testosterone receptor (or post-receptor), contributing to androgen resistance, can also cause significant abnormalities in sexual development.
In cases of 5-α-reductase deficiency, genotypic males can show a feminine appearance with ambiguous external genitalia throughout childhood. However, they may experience progressive virilization at puberty, due to increased testosterone levels. The development of facial hair and acne, both dependent on DHT production, is conspicuously absent. The condition is characterized by an abnormally high plasma testosterone-to-DHT ratio and may necessitate high-dose testosterone therapy to stimulate penile growth.
Although exceedingly rare, testosterone hypersecretion can occur due to the development of Leydig cell tumors in young children. This leads to a condition known as pseudoprecocious puberty and premature closure of the bony epiphyses, resulting in short stature. In such cases, treatment usually entails surgical removal of the testicular tumor, followed by replacement therapy if necessary.
Therapeutic Applications and Side Effects of Androgens
Androgens play a pivotal role in a multitude of therapeutic applications:
- One primary use is in hormone replacement therapy for adult males who have undergone castration or who suffer from hypogonadism due to testicular or pituitary insufficiency. In instances of pituitary insufficiency, androgen therapy typically begins in puberty, often in conjunction with continued growth hormone therapy, to promote growth spurt and normal development of sexual characteristics. It is crucial, particularly when treating boys, to carefully monitor treatment to prevent premature closure of the epiphyses. In general, androgens are not used to treat decreased libido and impotence, unless they are associated with confirmed hypogonadism.
- Androgens also function as protein anabolic agents, augmenting muscle mass following chronic debilitating or wasting disease, major surgery, or terminal conditions. Synthetic analogues, known as anabolic steroids, are often used due to their reduced androgenic effects and enhanced anabolic outcomes (eg, nandrolone or stanozolol). These anabolic actions contribute to bone remineralization, a significant benefit for androgen-deficient men with osteoporosis.
Although the use of anabolic steroids to treat aplastic anemia is a point of contention, it is still considered beneficial. However, its use by bodybuilders or athletes to increase aggressiveness, muscle strength, and athletic performance is unjustified and illegal, as high doses can severely impact the liver and cardiovascular system.
Erythropoietin (EPO), a hormone secreted primarily by kidney and liver cells, stimulates the formation of red blood cells from bone marrow precursor cells. Its synthesis is triggered by hypoxia in tissue and androgenic steroids. Recombinant human erythropoietin is now available to treat secondary anemia due to chronic renal failure.
- Androgens also play a role in treating estrogen-dependent tumors: Certain advanced breast and cervical cancers in women may regress when exposed to androgens. However, this treatment is accompanied by unavoidable masculinizing side effects, such as facial hair growth, interruption of the menstrual cycle, deepening of the voice, and acne development.
Androgen therapy is associated with certain adverse effects, including potential liver damage, liver tumors, and cholestatic jaundice. Weight gain due to sodium and water retention may occur, particularly in patients with heart or kidney disease. Other side effects include priapism (persistent abnormal erection) and a decrease in male fertility due to a negative feedback effect on GnRH and gonadotrophin release. However, suppression of spermatogenesis and decreased testicular size during treatment with these compounds are generally reversible upon therapy cessation.
Kindly Let Us Know If This Was helpful? Thank You!


