
The classification system for diabetes mellitus has undergone various iterations since the ADA workshop in 1993. Over time, there has been a better appreciation of the pathophysiologic basis of the various subtypes of diabetes mellitus. For this reason, the simple designation of diabetes as either insulin-dependent or independent is no longer recommended.
Type II diabetes mellitus (T2DM) accounts for 80-90% of all cases of diabetes mellitus. De Fronzo proposed the diabetes triumvirate which highlights the core pathophysiologic defects central to our understanding of type 2 diabetes mellitus. De Fronzo’s ominous octet subsequently supplanted the initial triumvirate of type 2 diabetes. In more recent times, additional pathophysiologic mechanisms have been recognized, leading to a more colorful successor to the ominous octet – “ the egregious eleven.” In this article, basic insulin physiology, pathophysiologic defects and mechanism of action of insulin in diabetes mellitus will be reviewed.
Insulin Physiology
Structure of insulin
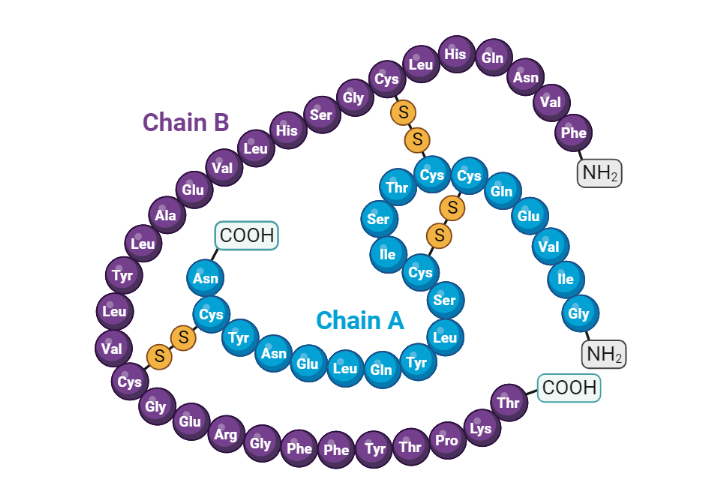
Insulin is a 51 amino acid peptide hormone that consists of two chains (A and B) linked by a pair of disulfide bonds. The A (21 amino acids) and B (30 amino acids) chains of insulin are linked by an intervening sequence of amino acids known as the connecting peptide (c-peptide)(1). The gene that encodes for human insulin produces an mRNA transcript that is translated into a large 110 amino acid polypeptide sequence known as preproinsulin. Preproinsulin then undergoes further processing in the lumen of the rough endoplasmic reticulum to produce proinsulin. Proinsulin is then ferried into the Golgi apparatus, where it is subsequently cleaved into the native insulin peptide and c-peptide (2). Along with these products of proinsulin processing, amylin and other intermediate peptides are packaged into secretory granules by the pancreatic beta cell(3).
The regulation of blood glucose
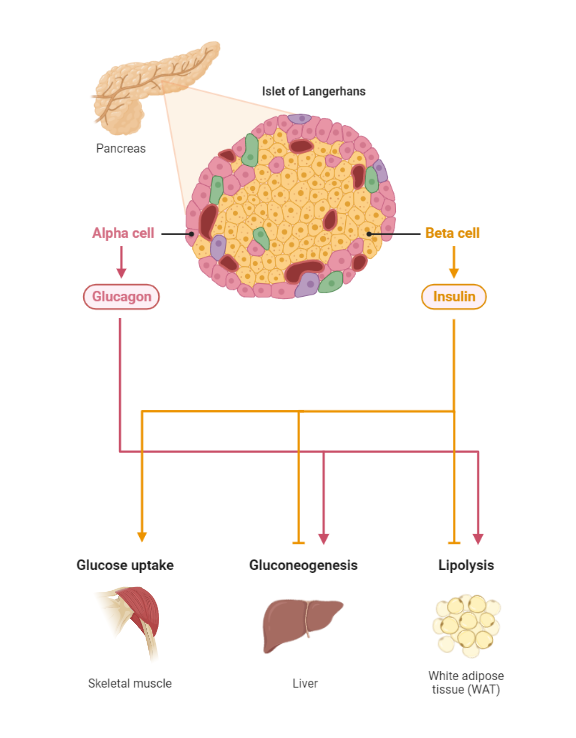
Glucose homeostasis depends on the action of insulin and a host of other counterregulatory hormones. This finely tuned system depends on the action of hormones, neural stimuli, cytokines, and other regulatory cytokines working together in various organs to control plasma glucose. Indeed, the pancreatic beta-cell is pivotal in directing the orchestra of this complex homeostatic system(4).
During the fasting state, the relative decline in insulin levels results in the oxidation of fatty acids in adipose tissue, making fatty acids a primary source of energy in the fasting state. For example, the liver utilizes fatty acids for gluconeogenesis during a prolonged fast. Conversely, the brain has obligatory glucose requirements making it necessary to have alternative sources of energy supply during a fast. The liver serves as a valuable source of glucose during a prolonged fast. Glucagon is released by alpha cells of the pancreas during a fast and promotes hepatic gluconeogenesis and glycogenolysis, thus maintaining plasma glucose concentration within a physiologic range.
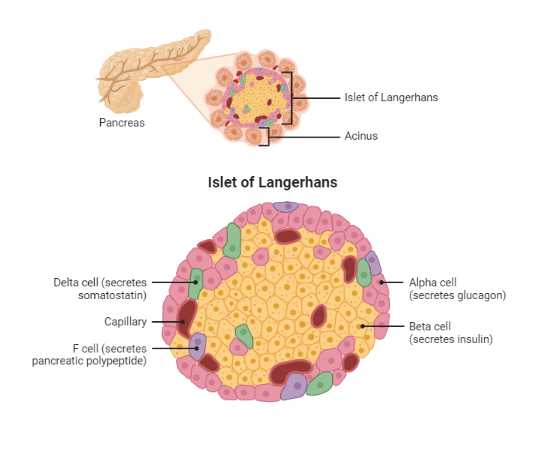
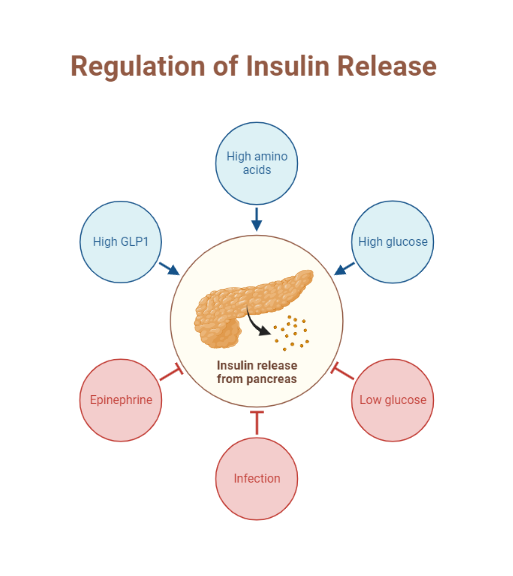
Conversely, during the postprandial state, glucose sensing by the pancreatic beta-cell results in insulin release. Insulin then exerts its metabolic action through various processes, including the inhibition of hepatic glucose output (reduced glycogenolysis and gluconeogenesis), the promotion of glucose uptake by peripheral tissues (muscle and adipose tissue), and a reduction in lipolysis (adipose tissue)(5) . Next, we will explore the effects of insulin on various tissues, including skeletal muscle, adipose tissue, and liver.
The effects of insulin on various tissues
Skeletal muscle relies on glucose and free fatty acids as energy sources in the postprandial and fasting states, respectively.
The skeletal muscle serves as the primary site of glucose uptake after ingestion of a meal (postprandial period), with insulin being the primary hormone responsible for this function. An increase in serum glucose after meal intake is sensed by pancreatic beta cells, which subsequently release insulin. Insulin then binds the insulin receptor and sets off a signaling cascade which results in the ferrying of glucose transporter 4 (also known as GLUT 4) from the sarcoplasm to the plasma membrane of the skeletal muscle (6,7) through a process of exocytosis(7). GLUT-4 then mediates the uptake of glucose by the skeletal muscle.
When glucose enters the myocyte of skeletal muscles, it is phosphorylated by the hexokinase enzyme into glucose-6-phosphate, which can be channeled into either glycogen synthesis (and storage) or utilized in the glycolytic pathway. A substantial quantity of glucose entering the glycolytic pathway is oxidized to produce energy, with only 10% being channeled into the production of lactate.
On the other hand, during the fasting state (sometimes referred to as the postabsorptive state), relatively low insulin levels impair the usual anti-lipolytic action of insulin in white adipose tissue. Consequently, increased lipolysis in white adipose tissue results in the production of fatty acids. These free fatty acids then become the primary source of fuel for skeletal muscle(8).
During the fasting state, the liver serves as the primary source of endogenous glucose production. This allows cells that can only utilize glucose as a primary source of energy, such as neurons, red blood cells, and renal medulla cells, to function optimally. The liver achieves this goal by increasing glycogenolysis, gluconeogenesis, and glycogen synthesis in the postabsorptive period. This complex system requires the interplay of various hormones (insulin, glucagon, catecholamines, glucocorticoids), substrates (glucose, glycerol), and allosteric factors (acetyl CoA, glucose, and glucose-6-phosphate)(9) beyond the scope of this text.
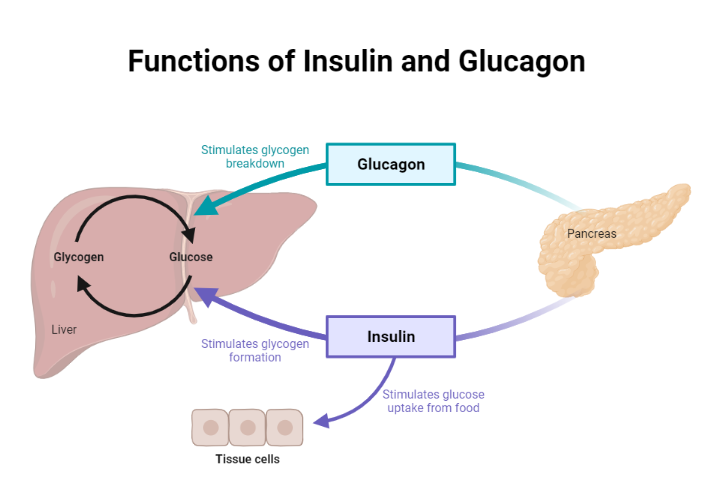
In the postprandial period, insulin mediates various processes that result in the suppression of glucose production by the liver.
Insulin, through its anti-lipolytic action, suppresses the breakdown of triglycerides into free fatty acids in white adipose tissue. Free fatty acid, a substrate for gluconeogenesis by the liver, is therefore reduced, resulting in the suppression of hepatic glucose production(9,10).
Glucagon stimulates hepatic glycogenolysis (increased hepatic glucose production). The presence of high levels of circulating insulin after a meal inhibits the release of glucagon by alpha cells of the pancreas(10), which consequently reduces hepatic glucose output.
The Insulin receptor
The insulin receptor (IR) has an extracellular portion composed of two alpha subunits and a transmembrane section comprising two beta subunits. In normal physiology, the alpha subunits inhibit the intrinsic tyrosine kinase activity of the beta subunits(11).
When insulin binds the alpha subunit of the IR, it suppresses the ability of the alpha subunit to inhibit the beta subunit. Consequently, insulin to IR binding promotes the phosphorylation of intracellular proteins required for various downstream signaling cascades in different tissues(12).
Mechanism of action of insulin in diabetes mellitus
Insulin resistance is central to our understanding of persistent hyperglycemia in patients with type 2 diabetes mellitus. It is characterized by an inability of normal plasma insulin to exert its glucose-lowering effects in the fed (postprandial) state. Consequently, increased hepatic glucose output increased lipolysis, and impaired peripheral glucose uptake leads to hyperglycemia(13).
Table 1. Mechanisms of insulin resistance in type 2 diabetes mellitus
| Tissue | Pathophysiologic defect | Result |
| Skeletal muscle | Impaired translocation of GLUT4 to plasma membrane of myocytes. Defective tyrosine phosphorylation of various proximal insulin receptor substrates. | Reduction in glucose uptake by skeletal muscle(5). |
| Adipose tissue | Reduced sensitivity of adipose to tissue to the anti-lipolytic action of insulin (an unknown underlying mechanism) Release of adipokines associated with insulin resistance. | Increased lipolysis(14) |
| Liver | Adipokines and free fatty acids (adipose tissue-derived) impair insulin signaling in the liver. | Impaired suppression of hepatic glucose output, which results in persistent hyperglycemia(15) |
Insulin therapy (practical pearls)
Insulin therapy may be either adjunctive or utilized primarily in managing type 2 diabetes mellitus. However, insulin therapy is required to prevent life-threatening ketoacidosis for patients with either anatomic or functional pancreatic insufficiency (e.g., type 1 diabetes mellitus).
Although guided by clinical evidence, the recommendations for insulin use in type 2 diabetes mellitus vary significantly across various clinical practice guidelines. It is, however, generally accepted that insulin is initiated in patients with anticipated beta cell failure and worsening hyperglycemia despite adherence to optimized doses of non-insulin therapies(16,17).
Patients with type 1 diabetes mellitus require both basal and prandial insulin if they are on multiple daily insulin injections. The usual starting total daily dose of insulin (TDDi) is 0.5units per kg body per day. The TDDi is split up into basal (40-60%) and bolus (40-60%) insulin. Patients with optimal carbohydrate counting skills may use a carbohydrate ratio to estimate the amount of bolus insulin(18).
Table 2. Pharmacodynamic profile of various insulins
| Type | Onset | Peak | Duration |
| Long-acting | |||
| Detemir U100 | 1-2 h | Mild effect at 4 h‡ | <24 h |
| Glargine U100 | 1 h | None | 24 h |
| Glargine U300 | 6 h | None | 24-36 h |
| Degludec U100/U200 | 1 h | None | 42 h |
| Intermediate-acting | |||
| NPH insulin U100 | 1-2 h | 4-14 h | 4-14 h |
| Bolus insulin | |||
| Lispro, Aspart, Glulisine U100 (rapid-acting) | 5-15 min | 0.5-1.5 h | 3-5 h |
| Fiasp (ultra-rapid acting) | 2.5-5 min | 1 h | 3-5 h |
| Regular human insulin (short-acting) | 30-60 min | 2-4 h | 6-12 h |
| Concentrated insulin Humulin R U500 | 6-10 h | ||
| Inhaled insulin | |||
| Afreeza | 5-15 min | 50 min | 3 h |
| Oral insulin | |||
| ORMD-0801‡‡ | – | – | – |
U100 refers to 100units of insulin in 1mL of liquid. U200 refers to 200units of insulin in 1 mL of liquid. U300 refers to 300units of insulin in 1mL of liquid. Derived from references (18–20)
‡‡ORMD-0801 is a potential oral insulin therapy for the management of type 2 diabetes. There are ongoing phase 3 trials of this medication(21).
Table 1. Pharmacokinetic Profile of Exogenous insulin
| Pharmacokinetic factor | Characteristics |
| Absorption | SC administration of exogenous insulin results in direct absorption into the bloodstream. The T50% has an intraindividual variability of 50% and an interindividual variability of 25%. |
| Distribution | The volume of distribution is dependent of the extracellular space. |
| Metabolism and Elimination | Degradation of exogenous insulin occurs in the kidney (~60%) and liver (~40%) after SC administration. |
SC Subcutaneous, T50% Coefficient of variation of absorption of 50% (Time for 50% of the administered dose to be absorbed), Endogenous insulin (50% of insulin delivered into the portal vein by the pancreas is metabolized in the liver). Derived from references (22–25)
Clinical Pearl
What are the factors associated with improved absorption of insulin administered subcutaneously?
- Exercise of a limb will improve insulin absorption within an hour of injection.
- Rubbing or massaging the site of injection
- Warm compresses, hot shower or bath improves accelerates insulin absorption.
- Site of injection : absorption is faster if insulin injected at abdominal compared to alternative sites.
Pathophysiology Pearl
What is the challenge in administering insulin via the oral route?
Endogenous insulin is secreted via either high-frequency pulses every 5-15 minutes or low-frequency ultradian pulses every 80-120 minutes. After an oral glucose challenge, about 40-80% of insulin released into the portal vein undergoes first pass metabolism in the liver. This phase of hepatic metabolism enables insulin to exert various effects on glucose metabolism, such as the promotion of glycogenolysis, inhibition of glycogenesis, increased gluconeogenesis, and a reduction in glucagon secretion(24).
Of all the routes of potential insulin administration (pulmonary, subcutaneous, rectal, ocular, vaginal), the oral route, is the closest to mimicking the portal-systemic blood insulin gradient seen with endogenous insulin. This is because orally administered insulin is delivered into the portal vein after absorption. Thus oral insulin administration mimics what exists physiologically. Unfortunately, insulin is a peptide hormone; hence it is subject to degradation in the harsh acidic pH of the stomach. Also, additional hindrances to oral insulin absorption such as enzymatic degradation, efflux pumps and epithelial barriers contribute to it’s poor bioavailability(26).
The pharmaceutical industry has attempted various means of delivering oral insulin. This has however remained a persistent challenge due to the reasons outlined above. Oramed Pharmaceuticals novel permeation enhancer technology aims to deliver insulin via a special enteric coated pill composed of bile salts, peptidase inhibitor, soyabean trypsin inhibitor, EDTA and omega-3 fatty acids. Its candidate oral insulin pill ORMD-0801 is now in phase 3 clinical trials(21).
References
1. Steiner DF, Park SY, Støy J, Philipson LH, Bell GI. A brief perspective on insulin production. Diabetes Obes Metab. 2009 Nov;11 Suppl 4:189–96.
2. Davidson HW. (Pro)Insulin processing: a historical perspective. Cell Biochem Biophys. 2004;40(3 Suppl):143–58.
3. Fu Z, Gilbert ER, Liu D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr Diabetes Rev. 2013 Jan 1;9(1):25–53.
4. Norton L, Shannon C, Gastaldelli A, DeFronzo RA. Insulin: The master regulator of glucose metabolism. Metabolism. 2022 Apr;129:155142.
5. Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018 01;98(4):2133–223.
6. Ramos PA, Lytle KA, Delivanis D, Nielsen S, LeBrasseur NK, Jensen MD. Insulin-Stimulated Muscle Glucose Uptake and Insulin Signaling in Lean and Obese Humans. J Clin Endocrinol Metab. 2021 Apr 1;106(4):1631–46.
7. Chang L, Chiang SH, Saltiel AR. Insulin Signaling and the Regulation of Glucose Transport. Mol Med. 2004 Jul;10(7):65–71.
8. Abdul-Ghani MA, DeFronzo RA. Pathogenesis of Insulin Resistance in Skeletal Muscle. Benian GM, editor. J Biomed Biotechnol. 2010 Apr 26;2010:476279.
9. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017 Oct;13(10):572–87.
10. Lewis GF, Carpentier AC, Pereira S, Hahn M, Giacca A. Direct and indirect control of hepatic glucose production by insulin. Cell Metab. 2021 Apr 6;33(4):709–20.
11. Boucher J, Kleinridders A, Kahn CR. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb Perspect Biol. 2014 Jan;6(1):a009191.
12. Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, et al. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr Rev. 2017 Jun 19;38(5):379–431.
13. Lee SH, Park SY, Choi CS. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J. 2022 Jan;46(1):15–37.
14. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016 Jun;125:259–66.
15. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009 Sep 1;42(13):1331–46.
16. Aschner P. Insulin Therapy in Type 2 Diabetes. Am J Ther. 2020 Feb;27(1):e79–90.
17. Meneghini LF. Insulin therapy for type 2 diabetes. Endocrine. 2013 Jun;43(3):529–34.
18. Janež A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, et al. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: a Narrative Review. Diabetes Ther. 2020 Feb;11(2):387–409.
19. Lamos EM, Younk LM, Davis SN. Concentrated insulins: the new basal insulins. Ther Clin Risk Manag. 2016 Mar 9;12:389–400.
20. An inhaled insulin (Afrezza). JAMA. 2015 Jun 2;313(21):2176–7.
21. King Hamad University Hospital, Bahrain. A Double-Blinded, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy and Safety of ORMD-0801 in Uncontrolled Type 2 DM Subjects on Diet Control Alone, Metformin Monotherapy, or Two or Three Oral Glucose-lowering Agents [Internet]. clinicaltrials.gov; 2021 Mar [cited 2022 Sep 9]. Report No.: NCT04817215. Available from: https://clinicaltrials.gov/ct2/show/NCT04817215
22. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998 Oct;19(5):608–24.
23. Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984 Sep;27(3):351–7.
24. Matteucci E, Giampietro O, Covolan V, Giustarini D, Fanti P, Rossi R. Insulin administration: present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug Des Devel Ther. 2015;9:3109–18.
25. Turnheim K, Waldhäusl WK. Essentials of insulin pharmacokinetics. Wien Klin Wochenschr. 1988 Feb 5;100(3):65–72.
26. Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011 Dec;32(36):9826–38.
Kindly Let Us Know If This Was helpful? Thank You!

