Physiology of Renal Glucose Handling by SGLT-2 transporters
Plasma glucose filtration occurs at the glomerulus, after which its re-absorption is carried out within the proximal tubule through intricate mechanisms facilitated by a number of transporters. The protein known as sodium-glucose cotransporter 2 (SGLT2), which is predominantly found on the apical membrane of the S1 and S2 segments, plays a key role in facilitating most of this glucose reabsorption.
In addition to SGLT2, the sodium-glucose cotransporter 1 (SGLT1) protein helps to reabsorb residual glucose, specifically within the S3 segment of the apical membrane of the proximal tubule. The reabsorption process for both glucose and sodium is primarily driven by the electrochemical potential gradient of sodium, the maintenance of which is ensured by the Na+/K+ pump located within the basolateral membrane.
Following re-absorption, the accumulated glucose within the epithelium is then transported into the bloodstream. This transport is facilitated by the GLUT2 protein, which is located within the basolateral membrane.
Mechanism of Action
SGLT-2 inhibitors operate by impeding the function of the sodium-glucose cotransporter-2 protein (SGLT-2) within the kidneys. This protein is primarily tasked with the re-absorption of glucose from the glomerular ultrafiltrate. By inhibiting SGLT-2, these drugs lead to increased glucose excretion in urine and consequentially lower blood glucose levels.
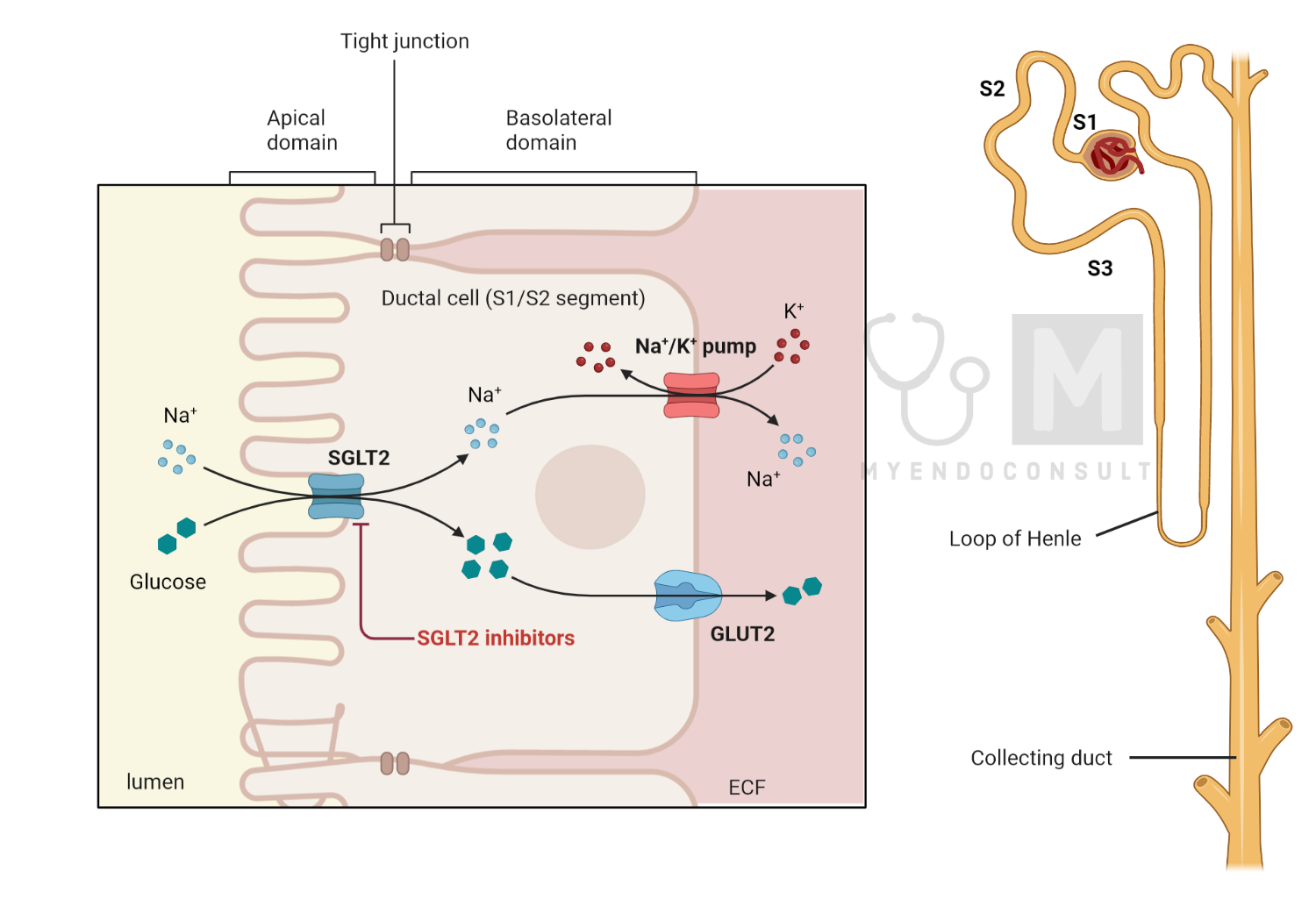
Specific SGLT-2 inhibitors, such as dapagliflozin, canagliflozin, and empagliflozin, act primarily by preventing glucose reabsorption within the S1 and S2 segments of the proximal tubule, the primary locations of SGLT-2 expression. The S3 segment of the proximal tubule, where SGLT-1 is predominantly expressed, is responsible for reabsorbing residual glucose from the glomerular filtrate. However, since SGLT-1 has a reduced glucose transport capacity compared to SGLT-2, its inhibition by SGLT-2 inhibitors is generally considered clinically insignificant.
The ultimate result of the inhibition of SGLT-2 by these drugs is a reduction in the reabsorption of glucose into the bloodstream, which contributes to lower blood glucose levels.
Practice Guide
The prescribing guidelines for some commonly used SGLT2 inhibitors are as follows.
- For Dapagliflozin (Farxiga), the recommended starting dose is 5 mg once a day, with a maximum dose of 10 mg once a day.
- Canagliflozin (Invokana) has a recommended starting dose of 100 mg once daily and a maximum dose of 300 mg once daily.
- Empagliflozin (Jardiance) should start at a dose of 10 mg once daily, and the maximum dose is 25 mg once a day.
- Ertugliflozin (Steglatro) is recommended to start at 5 mg once daily, with a maximum dose of 15 mg once daily.
Although SGLT2 inhibitors are generally considered safe and effective for the treatment of type 2 diabetes, it is crucial to recognize possible side effects and necessary precautions. These medications may cause urinary tract infections, dehydration, and diabetic ketoacidosis (DKA), among other effects.
Frequently Asked Questions
Mechanism of action of Jardiance in Heart failure
Empagliflozin, commonly known by its brand name Jardiance, is an SGLT2 inhibitor originally developed to manage type 2 diabetes mellitus. Interestingly, it has recently shown benefits in the treatment of heart failure, regardless of whether the patients have diabetes or not.
One proposed mechanism involves diuresis and natriuresis. By inhibiting the SGLT2 protein in the proximal renal tubules, empagliflozin improves glucose and sodium into the urine, leading to glycosuria and natriuresis. This action results in a reduction in plasma volume, thus decreasing the preload, which is essentially the volume of blood that fills the heart prior to contraction. This decrease in preload could help alleviate heart failure symptoms associated with volume overload, such as breathlessness and edema.
In addition to the diuretic effect, empagliflozin can also have an impact on cardiac fuel metabolism. It is suggested that empagliflozin could shift the primary energy source of the heart from glucose to ketone bodies and free fatty acids, which are considered more efficient fuels. This metabolic change could potentially enhance cardiac efficiency and function.
Furthermore, empagliflozin might contribute to a decrease in arterial stiffness and blood pressure. By reducing arterial stiffness and lowering blood pressure, empagliflozin could reduce afterload, which is the resistance that the heart must overcome to eject blood. This could potentially lead to an improvement in overall cardiac function.
Finally, empagliflozin has been suggested to exert anti-inflammatory and antifibrotic effects. By reducing systemic inflammation and fibrosis, known contributors to heart failure progression, empagliflozin could potentially slow disease progression.
Collectively, these mechanisms can contribute to the overall benefits of empagliflozin in heart failure, including reductions in hospitalizations due to heart failure and a possible decrease in cardiovascular mortality. However, it is essential to note that more research is needed to fully elucidate the underlying mechanisms. As of my last update in September 2021, ongoing studies are expected to shed more light on this.
Mechanism of renal benefits of Jardiance
First, empagliflozin acts to lower glomerular hyperfiltration, a condition often seen in the early stages of CKD and diabetes, where the kidneys filter blood at an abnormally high rate. This condition puts additional stress on the kidneys. By inhibiting SGLT2, empagliflozin increases glucose and sodium, reducing intraglomerular pressure, and thereby mitigating hyperfiltration.
Second, by promoting mild osmotic diuresis and natriuresis (excretion of sodium in urine), empagliflozin can reduce volume overload and blood pressure, factors that often exacerbate kidney damage. Lower blood pressure can ease the strain on the blood vessels, potentially slowing the progression of kidney damage.
Third, empagliflozin might exert beneficial effects through metabolic and hormonal pathways. It is thought that it can shift metabolism toward more efficient use of lipids for energy and may also have an impact on hormones such as insulin and glucagon, which have indirect effects on kidney function.
Finally, empagliflozin may reduce inflammation and fibrosis, both of which contribute to the progression of chronic kidney disease.
It is important to note that the renal benefits of empagliflozin seem to go beyond mere glucose control, as seen in trials with patients without diabetes. More research is ongoing to fully understand how empagliflozin and other SGLT2 inhibitors protect the kidneys.
References
- Ghezzi C, Loo DDF, Wright EM (2018) Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61:2087–2097
- Ferrannini E (2017) Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell Metab 26:27–38
- Wright EM (2021) SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2:2027–2037
- Nair S, Wilding JPH (2010) Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 95:34–42
- Fonseca-Correa JI, Correa-Rotter R (2021) Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front Med 8:777861
- Vallon V, Verma S (2021) Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu Rev Physiol 83:503–528
- Padda IS, Mahtani AU, Parmar M (2023) Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. StatPearls
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR (2017) Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 377:644–657
- Wiviott SD, Raz I, Bonaca MP, et al (2019) Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380:347–357
Kindly Let Us Know If This Was helpful? Thank You!


