The mechanism of action of temozolomide and its clinical application in the management of prolactinomas will be reviewed.
Physiology
Cellular protective mechanisms from mutagens : Endogenous and exogenous factors can promote DNA damage and inadvertently set off a cascade of events that leads to the formation of tumors. Cells in normal physiology maintain their integrity through a myriad of protective pathways such as mismatch repair, nucleotide excision repair, and methylguanine-DNA methyltransferase (MGMT) enzymatic processes(1).
Mechanism of action
Temozolomide (TMZ) is an alkylating chemotherapeutic agent that promotes the methylation of specific guanine (position O-6) and purine (positions N3 and N7) residues in DNA. This introduces breaks in the DNA of rapidly growing cells, like lactotroph tumors, leading to their apoptosis (programmed cell death)(2).
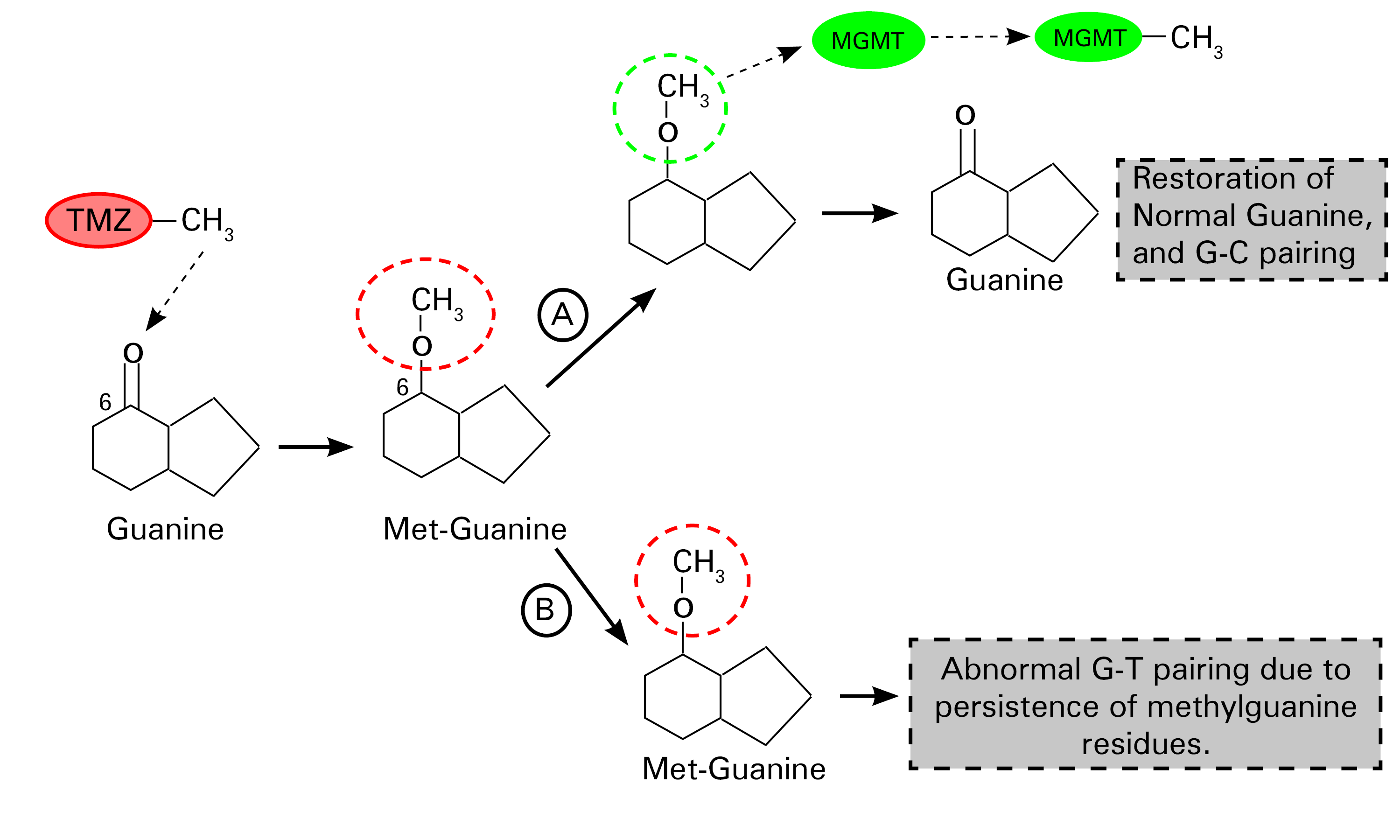
Fig. 1.0 Schematic representation of the mechanism of action of temozolomide.
TMZ promotes the methylation of guanine(G) at the number 6 carbon position, a step that leads to the formation of methylguanine residues in DNA. There is a “suicide enzyme” called methylguanine-DNA methyltransferase (MGMT), whose primary role is to remove these abnormal methyl groups, thus restoring the integrity of guanine residues in DNA (Step A). This defective methylated guanine pairs with thymine (T) instead of cytosine (C) (by convention) during replication. Mismatch repair enzymes excise these mispaired guanine-thymine residues, although this ultimately is an exercise in futility. Continuous cycles of erroneous G and T pairing and T excisions lead to irreparable DNA breaks that promote cell death (step B) (2). Redrawn and modified from Zhang J et al. (2012) Temozolomide: mechanisms of action, repair, and resistance. Curr Mol Pharmacol 5:102–114
Practice Guide
- An assessment of MGMT promoter methylation status is a useful predictive biomarker(3) in patients with aggressive prolactinomas or carcinomas(4). There is an inverse relationship between tumor levels of MGMT and the degree of responsiveness to temozolomide(4). Although MGMT as a biomarker is a novel approach to predicting response to TMZ, therapeutic response after a minimum of 3 cycles of treatment fared better than tumor levels of MGMT in a large cohort of patients with pituitary tumors (including prolactinomas)(5).
- TMZ being a chemotherapeutic agent is associated with expected short-term toxicity concerns such as nausea, emesis, and fatigue(6). Cytopenias (hematologic toxicity), requiring dose reduction or cessation of therapy, are however infrequent(5,6). Safety data after 5 to 8 years of exposure to TMZ is very reassuring, making it a valuable long-term salvage therapeutic option(7).
Clinical Trial Evidence
There are no published randomized trials evaluating the safety and efficacy of temozolomide compared to placebo. A recent systematic review of all published case reports and case series provided some meaningful insight into the response of dopaminergic agonist-resistant prolactinomas to temozolomide(6).
Key Message
Temozolomide has a favorable side effect profile and is a reasonable rescue therapeutic option in patients who have exhibited a suboptimal response to DAs, radiation therapy, or surgery. Approximately 75% of patients with treatment resistant prolactinomas achieved a reduction in tumor volume and serum prolactin levels after a trial of temozolomide.
This was a meta-analysis of case series and reports evaluating tumoral response to temozolomide in 42 subjects with either a prolactin-secreting adenoma or carcinoma. Patients with resistance to dopaminergic agonist therapy received oral temozolomide dosed as 150-200mg/m2 in 1 to 24 therapeutic cycles. The primary outcome was defined as a change in tumor size and improvement in hyperprolactinemia. A significant reduction in tumor size and prolactin levels, compared to baseline, occurred in 76.5% and 75% of patients, respectively(6).
References
- Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K. Role of MGMT in Tumor Development, Progression, Diagnosis, Treatment and Prognosis. Anticancer Res. 2009 Oct 1;29(10):3759–68.
- Zhang J, Stevens MFG, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012 Jan;5(1):102–14.
- Cankovic M, Nikiforova MN, Snuderl M, Adesina AM, Lindeman N, Wen PY, et al. The Role of MGMT Testing in Clinical Practice: A Report of the Association for Molecular Pathology. J Mol Diagn. 2013 Sep 1;15(5):539–55.
- Whitelaw BC, Dworakowska D, Thomas NW, Barazi S, Riordan‐Eva P, King AP, et al. Temozolomide in the management of dopamine agonist–resistant prolactinomas. Clin Endocrinol (Oxf). 2012;76(6):877–86.
- Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, et al. Temozolomide Treatment in Aggressive Pituitary Tumors and Pituitary Carcinomas: A French Multicenter Experience. J Clin Endocrinol Metab. 2010 Oct 1;95(10):4592–9.
- Almalki MH, Aljoaib NN, Alotaibi MJ, Aldabas BS, Wahedi TS, Ahmad MM, et al. Temozolomide therapy for resistant prolactin-secreting pituitary adenomas and carcinomas: a systematic review. Horm Athens Greece. 2017 Apr;16(2):139–49.
- Khasraw M, Bell D, Wheeler H. Long-term use of temozolomide: Could you use temozolomide safely for life in gliomas? J Clin Neurosci. 2009 Jun 1;16(6):854–5.
Kindly Let Us Know If This Was helpful? Thank You!


