The cytochrome P450 (CYP) superfamily of enzymes is responsible for the oxidative and reductive metabolic transformation of a wide range of xenobiotics, including medications used in clinical practice. CYP enzymes are involved in many clinically relevant drug-drug interactions, which can significantly impact the efficacy and safety of pharmacotherapy. This article aims to provide an in-depth understanding of CYP3A4 inhibitors, their interactions with substrates of CYP3A4, and implications for drug development and clinical practice.
CYP3A4: Prevalence, Significance, and Mechanisms of Action
CYP3A4 is the most abundant CYP enzyme in the liver and intestines, accounting for over 50% of the metabolism and elimination of medications available in the market. Indeed the activity of CYP3A4 can be modulated by various factors, including genetic polymorphisms, environmental influences, and the presence of other medications. CYP3A4 activity can be induced (accelerated) or inhibited (decreased), leading to alterations in drug concentrations and pharmacokinetic profiles.
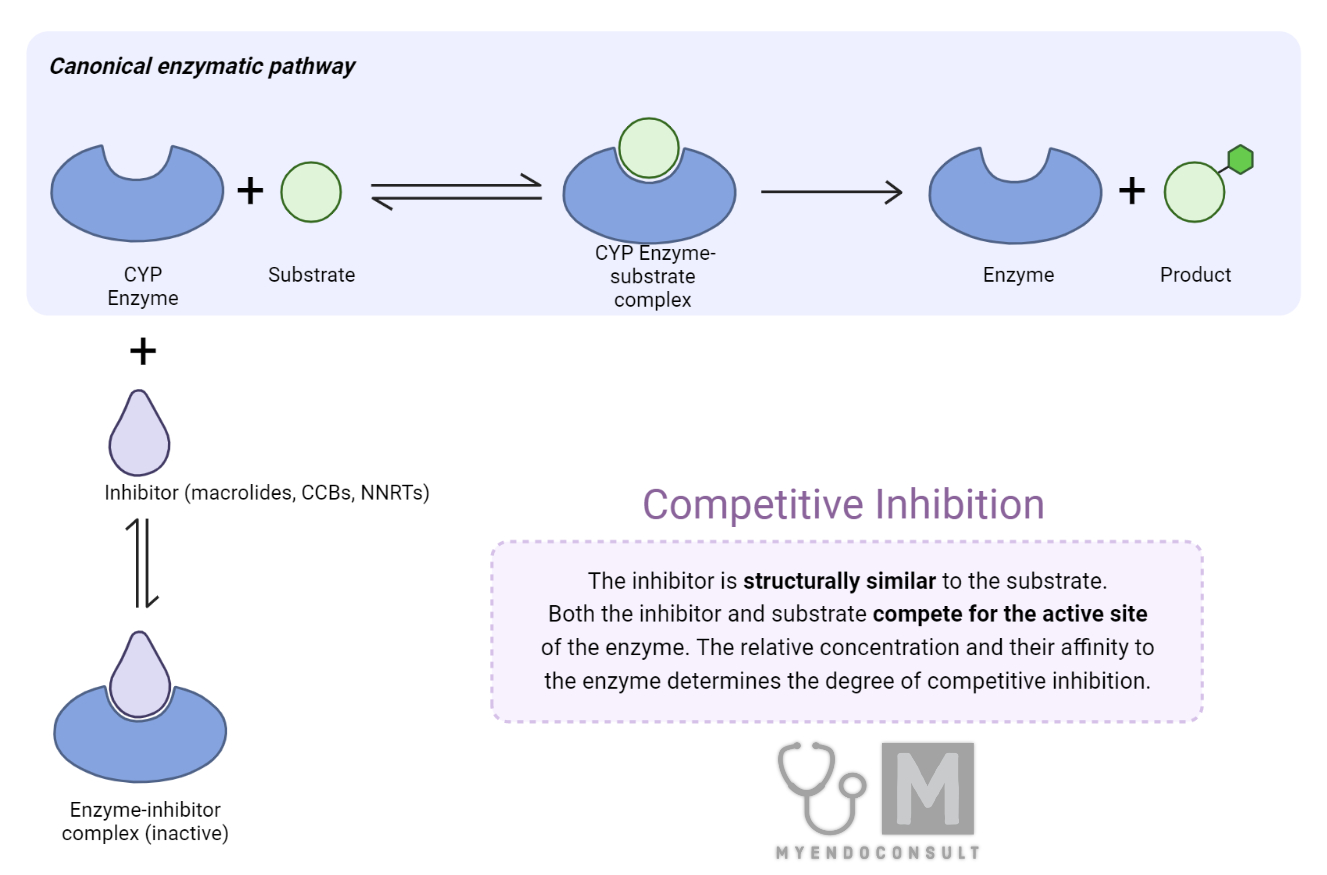
It is worth noting that the inhibition of CYP3A4 can result in the accumulation of parent drug concentrations, increasing the risk of adverse effects and potential toxicity. This can be particularly concerning when the drug in question has a narrow therapeutic index, or when patients are on multiple medications that are substrates of CYP3A4. In such cases, understanding the molecular mechanisms of CYP3A4 inhibition, the structure-activity relationships of inhibitors, and the potential for clinically relevant drug-drug interactions becomes crucial for drug development and optimization of pharmacotherapy.
Types Of CYP3A4 Inhibitors
The potency of CYP3A4 inhibition can be evaluated using pharmacokinetic parameters such as the area under the curve (AUC) and clearance. The AUC represents the total exposure of a drug in the systemic circulation over time, whereas clearance refers to the volume of blood or plasma that is cleared of the drug per unit time. When a CYP3A4 inhibitor is co-administered with a substrate drug, the inhibition of CYP3A4-mediated metabolism can lead to changes in these parameters.
| Drug Group | Weak CYP34A4 inhibitors (increase in AUC is under 2 fold. Clearance reduced by 20-50%) | Moderate CYP3A4 inhibitors (increase in AUC is 2-5x. Clearance reduced by 50-80%) | Strong CYP3A4 inhibitors (increase in AUC is >5 fold. Clearance reduced by >80%) |
|---|---|---|---|
| Antibiotics | Erythromycin | clarithromycin | |
| Antidepressants | Nefazodone | ||
| Azole Antifungals | Fluconazole, Miconazole | Itraconazole, Ketoconazole | |
| Calcium channel blockers | Diltiazem, Verapamil | ||
| H2 Receptor blockers | Cimetidine | ||
| NNRT inhibitors (ART) | Delavirdine | ||
| Protease inhibitors (ART) | Amprenavir | Indinavir, lopinavir, ritonavir, darunavir | |
| Plant based supplements | Grapefruit juice, Echinacea angustifolia, wild cherry, licorice |
In the context of CYP3A4 inhibition, an increase in the AUC of a substrate drug indicates that the drug's metabolism has been reduced due to the presence of the inhibitor. As a result, the total exposure of the drug in the systemic circulation is increased, which may lead to elevated drug concentrations and an increased risk of adverse effects or toxicity.
On the other hand, the clearance of the substrate drug is expected to decrease in the presence of a CYP3A4 inhibitor. Reduced clearance means that the drug is being eliminated from the body at a slower rate, which can also contribute to increased drug concentrations and potential adverse effects.
Common CYP3A4 Inhibitors: Molecular Basis and Clinical Relevance
A comprehensive understanding of the molecular basis of CYP3A4 inhibition is essential for predicting drug-drug interactions and designing drugs with reduced potential for such interactions. Here, we discuss the most common medications known to inhibit CYP3A4, highlighting their molecular basis for inhibition and clinical relevance.
Macrolide antibiotics, such as erythromycin and clarithromycin, are potent inhibitors of CYP3A4. They act by forming stable complexes with the CYP3A4 active site, preventing the binding and metabolism of substrate drugs. Azithromycin, however, is an exception within this class and does not exhibit significant CYP3A4 inhibition. This difference in inhibition potential is attributed to structural variations in the macrolactone ring, which impacts the binding affinity to CYP3A4.
Also, when it comes to calcium channel blockers, only the non-dihydropyridine class, including verapamil and diltiazem, are known to inhibit CYP3A4. They bind to the enzyme's active site, hindering access of substrate drugs. In contrast, dihydropyridine calcium channel blockers, such as amlodipine and nifedipine, do not exhibit significant CYP3A4 inhibition due to differences in their chemical structures.
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) used in the management of HIV exhibit diverse effects on CYP3A4 activity. Delavirdine is a potent inhibitor of CYP3A4, while other NNRTIs, such as efavirenz and nevirapine, are considered inducers. This variation in CYP3A4 modulation is attributed to differences in the chemical structures and binding affinities of these compounds to the enzyme's active site. The clinical implications of these interactions are particularly important in HIV-positive patients, as many antiretroviral medications are substrates of CYP3A4 and their pharmacokinetics can be significantly affected by the concurrent use of NNRTIs.
Azole antifungal agents, such as ketoconazole, itraconazole, and fluconazole, are also known to inhibit CYP3A4 by coordinating with the heme iron in the enzyme's active site. This interaction disrupts the catalytic cycle of CYP3A4, leading to reduced metabolism of substrate drugs. The clinical relevance of these interactions is particularly significant in patients receiving azole antifungals concomitantly with medications that have a narrow therapeutic index and are metabolized by CYP3A4, such as immunosuppressants and certain antiarrhythmics.
Protease inhibitors used in the management of HIV infection, such as ritonavir, lopinavir, and atazanavir, are potent CYP3A4 inhibitors. They act by binding to the enzyme's active site, which prevents the metabolism of substrate drugs. The inhibition of CYP3A4 by protease inhibitors can lead to significant drug-drug interactions, particularly with other antiretroviral medications, necessitating careful dose adjustments and monitoring of drug levels to avoid toxicity and maintain therapeutic efficacy.
Strategies to Minimize Clinically Relevant Drug Interactions
To reduce the risk of adverse effects and optimize pharmacotherapy, several strategies can be employed to minimize the impact of CYP3A4 inhibitors on drug metabolism:
- When possible, select alternative medications that are not substrates of CYP3A4 or are less prone to inhibition, thus reducing the potential for drug-drug interactions.
- When CYP3A4 inhibitors are used concomitantly with substrate drugs, dose adjustments may be necessary to account for altered pharmacokinetics and minimize the risk of adverse effects.
- In cases where dose adjustments are not feasible or the drug has a narrow therapeutic index, therapeutic drug monitoring can help optimize treatment by ensuring that drug concentrations remain within the therapeutic range.
- In some cases, genetic polymorphisms in CYP3A4 can influence an individual's response to medications and susceptibility to drug-drug interactions. Pharmacogenetic testing can help identify patients who may require dose adjustments or alternative medications due to altered CYP3A4 activity.
Conclusion
A comprehensive understanding of CYP3A4 inhibitors, their molecular mechanisms, and the potential for clinically relevant drug-drug interactions is crucial for drug development and optimal pharmacotherapy. By considering the pharmacokinetic properties and potential interactions of these medications, researchers and clinicians can better design drugs with reduced potential for interactions, manage drug therapy, and reduce the risk of adverse effects in patients.
References
- Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet 2007;46:681-96.
- Rendic S, Ci Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 1997;29:413-580.
Kindly Let Us Know If This Was helpful? Thank You!


