What is the Menstrual Cycle?
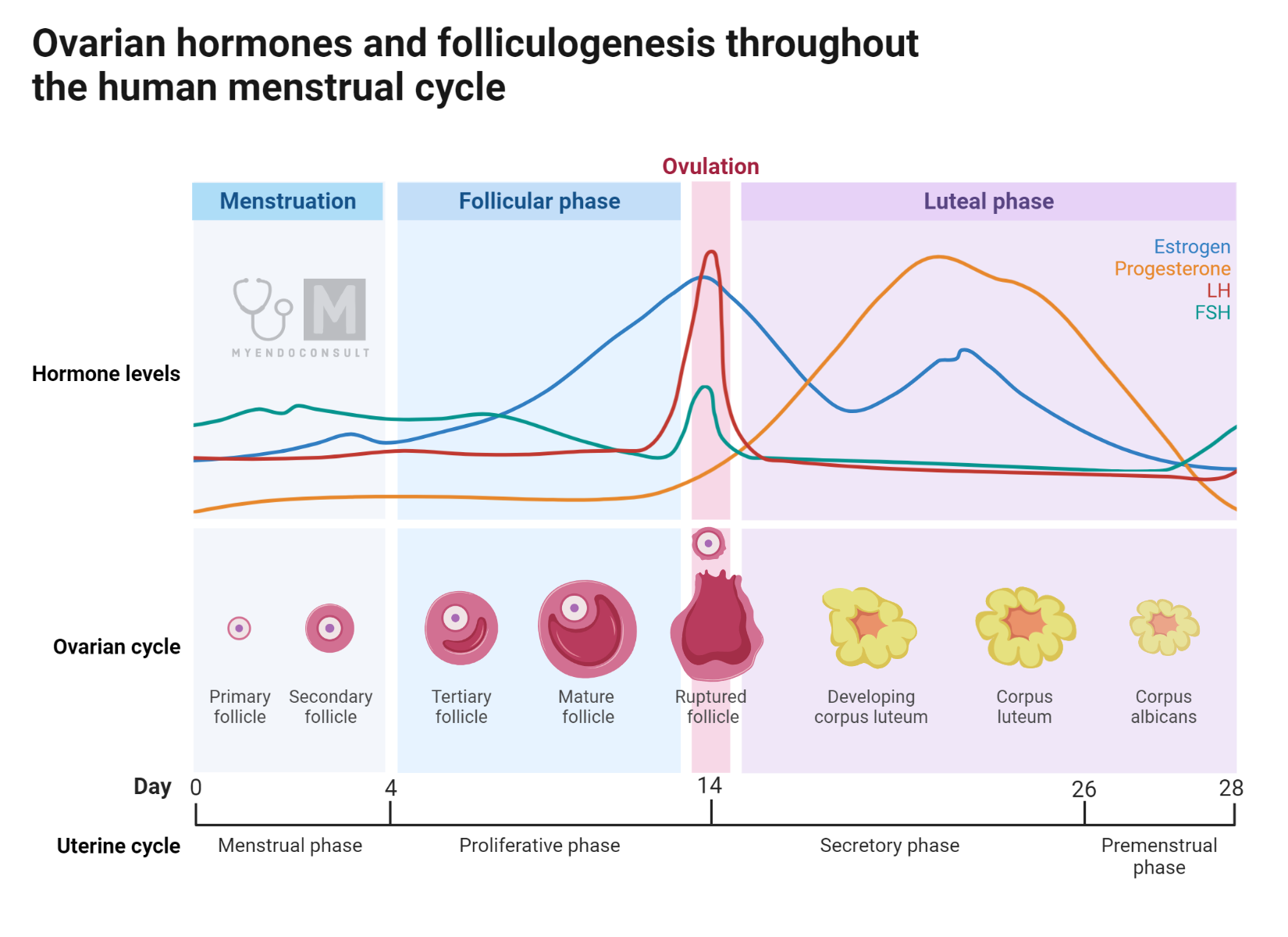
The menstrual cycle begins on the first day of vaginal bleeding, which occurs due to the shedding of the endometrium built during the previous cycle in the absence of conception. At this point, hormone levels of estradiol, progesterone, and inhibin are at a low, causing minimal ovarian negative feedback. This scenario results in an increase in pituitary secretion of Follicle Stimulating Hormone (FSH). FSH has various effects, including stimulating the growth and division of granulosa cells, enhancing aromatase activity, and promoting the production of inhibin and Luteinizing Hormone (LH) receptors.
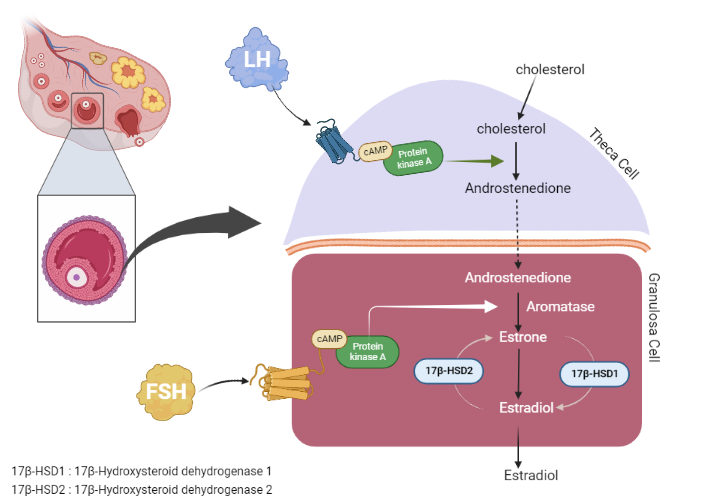
Aromatase activity is crucial for converting androgens into estrogens. FSH also stimulates the production of inhibin B, which subsequently inhibits FSH production. Moreover, FSH encourages the formation of gap junctions in the dominant follicle, which intensifies the interaction between the oocyte and cumulus cells.
The level of FSH in the body can influence the number of follicles that have the potential to mature into a Graafian follicle. As the selected follicle develops, the production of inhibin B escalates, which then suppresses FSH secretion by the pituitary gland. This sequence of events results in the growth of the most FSH-sensitive follicle, known as mono follicular growth. When a follicle reaches dominance, it inhibits the FSH sensitivity of other growing follicles through yet unidentified mechanisms. The oocyte’s communication with its environment through paracrine signaling likely plays a crucial role in follicle development. The oocyte with the most effective communication with its surroundings has a higher chance of survival. It is speculated that inhibin B plays a significant role in this process.
In the process of follicle development, the role of inhibin B is crucial, especially when it comes to follicle selection. In this stage, the follicle’s diameter is between 10-12mm. As the levels of estrogen start to rise a few days into this phase, there’s a corresponding decline in FSH levels, along with an increase in inhibin B concentration.
Inhibin B is a more powerful suppressor of FSH than estrogens. This hormonal signaling needs to be transported via the blood to reach the other ovary. Keeping FSH levels high, as seen in ovarian hyperstimulation protocols used in assisted reproductive techniques, doesn’t increase the pool of follicles but helps in protecting them from apoptosis, thus allowing more than one follicle to mature into a Graafian follicle.
The process of ovulation
Ovulation marks the completion of successful follicle development. It involves the release of the oocyte granulosa cumulus cells from the follicle. The remaining granulosa cells and theca cells undergo luteinization to form the corpus luteum. Ovulation is triggered by the LH surge, with the interval between the start of the LH surge and ovulation being approximately 36-42 hours.
The factors and processes that lead to the LH surge aren’t entirely clear. While low levels of estrogen and progesterone suppress LH secretion, an increase in these hormones, particularly a slight increase in progesterone during the end of the follicular phase, considerably augments the pituitary’s sensitivity to GnRH, thus triggering an LH surge. Progesterone, produced in minimal amounts during the follicular phase under the influence of LH, plays a vital role in this process.
The mid-cycle LH surge, which is crucial for timing ovulation, results from the increase in pituitary sensitivity to GnRH, a response to positive feedback signals from the mature follicle ready for ovulation. The LH surge is also accompanied by an FSH surge, the role of which is still not entirely understood.
The role of a hypothalamic neuropeptide called kisspeptin is significant in the regulation of GnRH. It stimulates LH secretion through GnRH and regulates a GnRH surge followed by an LH surge in many species. However, its role in humans is still unclear.
The LH surge has various effects, such as closing the gap junction between the oocyte and the granulosa cumulus cells, and reducing cAMP levels in the oocyte, thus allowing the completion of the first meiosis of the oocyte that began during fetal life.
The timing of this process is critical to have a fertilizable oocyte available upon release. The LH surge also triggers the release of proteolytic factors leading to follicular rupture and the release of the oocyte cumulus cell complex in a process that takes 2-5 minutes. Inflammatory cells and proteolytic factors act to weaken the epithelial surface over the follicle, while increased blood flow and edema create the intrafollicular pressure necessary for the oocyte cumulus complex to exit the follicle.
Events after ovulation (corpus luteum formation)
The corpus luteum, a temporary endocrine gland, is composed of luteinized granulosa and theca cells, as well as endothelial, immune, and fibroblast cells. This gland is a significant source of estrogens, inhibin A, and more specifically, progesterone.
Following the LH surge, there is a substantial increase in progesterone production. This is primarily due to the theca cells, which already contain the enzymatic system responsible for progesterone synthesis (StAR, CYP11A1, and 3βHSD) at the time of the LH surge. The LH surge enhances StAR expression in the luteal granulosa cells and aids the formation of the rest of the enzymatic system. In combination with the vascularization of the entire corpus luteum, this facilitates progesterone production in the luteal granulosa cells as well.
Luteal theca cells continue to be the primary producers of androgens, which are subsequently converted into estrogens in the luteal granulosa cells. Therefore, the two-cell system for estrogen production remains active following the formation of the corpus luteum. Notably, both the luteal theca cell and the luteal granulosa cell are stimulated by LH.
LH is vital for the formation and maintenance of the corpus luteum. Nonetheless, LH cannot stop the eventual regression of the corpus luteum. If conception does not occur during the cycle, the corpus luteum disintegrates in the absence of placental chorionic gonadotropin (HCG), leading to a loss of structural and functional cohesion.
This process is likely due to the diminishing responsiveness of the aging corpus luteum to LH. As the corpus luteum ages, there is a decrease in StAR expression and progesterone production, which is preceded by the death of the corpus luteum cells. When progesterone levels fall, the endometrium can no longer be sustained, leading to menstrual shedding and the start of a new menstrual cycle.
However, if conception takes place, the corpus luteum is preserved by HCG, which is produced by the trophoblasts. The progesterone produced by the corpus luteum is crucial for preparing the endometrium for implantation and sustaining an intrauterine pregnancy until the placenta becomes functional, which typically occurs around the 12th week of gestation.
Kindly Let Us Know If This Was helpful? Thank You!


