In patients with thyrotoxicosis or thyroid storm, characterized by symptomatic hyperthyroidism, there is an excess of thyroid hormone production and secretion. This can lead to increases in heart rate, tremors, and nervousness. Propranolol, a nonselective beta-1 and beta-2-blocker, is the most studied and commonly used treatment option for this condition. It can address increased heart rate and tremors and can reverse reduced systemic vascular resistance while inhibiting peripheral conversion of T4 to the more biologically active hormone, T3. This article reviews the current evidence for the use of propranolol in this clinical setting.
What is Thyroid Storm?
Thyroid storm, is a severe and potentially life-threatening condition that results from excessive levels of thyroid hormones, thyroxine (T4) and 3,5,3'-triiodothyronine (T3), in the bloodstream. It is characterized by symptomatic hyperthyroidism, leading to a variety of symptoms such as tachycardia, palpitations, tremors, unintentional weight loss, diarrhea, and nervousness. The primary treatment approach for this condition involves using medications that inhibit the synthesis of T4 and T3 and reduce their peripheral conversion.
Propranolol, a non-selective beta-1 and beta-2-blocker, has emerged as the most studied and commonly used treatment option for thyrotoxicosis and thyroid storm.
Mechanisms of thyroid hormone metabolism
The most significant pathway that metabolizes thyroid hormones and regulates T3 bioavailability in human tissues is deiodination. The thyroid gland produces only a small amount of T3, while most of the T3 (roughly 80%) in peripheral tissues is produced by the enzymatic outer ring deiodination (ORD) of T4. Similarly, thyroidal secretion of the metabolite rT3 is minimal and is produced by the inner ring deiodination (IRD) of T4. Besides deiodination, other routes might also be important in specific situations.
For example, sulfoconjugation of iodothyronines is a crucial pathway in the development of animals. The process of sulfation is catalyzed by a family of enzymes i.e., sulfotransferases, and it involves the transfer of the sulfonate group from the 3-phosphoadenosine 5-phosphosulfate (PAPS), to the phenolic hydroxyl group of iodothyronines. Due to the sulfoconjugation of thyroid hormones (T4 and T3) and their metabolites (rT3 and 3,3-T2), iodothyronines may degrade even faster.
In addition to deiodination, the sulfation of thyroid hormones can further regulate the bioavailability of THs, particularly in developing tissues. Furthermore, to restore their bioactivities, tissue sulfatases can convert sulfated T3, rT3, or 3,3-T2 to the parent precursors.
Another important metabolic pathway, particularly for the metabolism of T4, involves the phenolic conjugation of thyroid hormones with glucuronic acid to create their glucuronides. Glucoronidated T4 (T4G) has a greater volume of distribution than T4 since it is a more polar molecule.
The liver is the main site of glucuronidation, where it may occur before biliary-fecal excretion from glucuronides of thyroid hormone. The glucuronidation of T3 is less significant quantitatively and physiologically than that of T4. Glucuronidase in intestinal bacteria catalyzes deconjugation back to T4 in the intestinal lumen. When this recovered T4 is absorbed through the intestinal tract, the hormone re-enters the portal circulation and is once again available to the liver. Furthermore, T4 can be delivered to intracellular locations through deconjugation at tissue sites.
In effect, the metabolism of thyroid hormones by deiodinase enzymes causes the activation or inactivation of thyroid hormones. Conversely, metabolism by sulfotransferases, glucuronidases or sulfatases changes the distribution, solubility and biological potency of thyroid hormones.
Pathophysiology of thyrotoxicosis
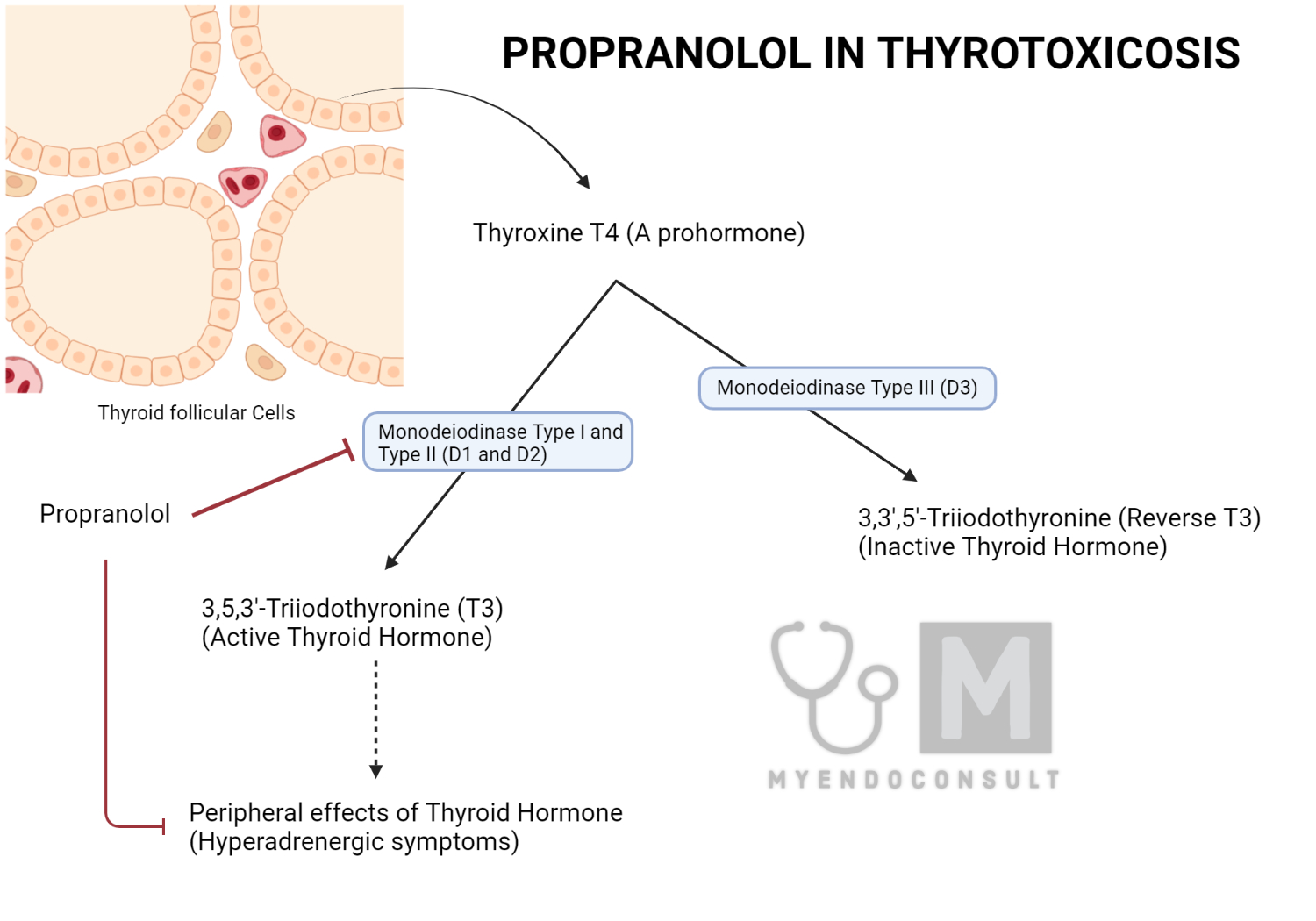
Thyrotoxicosis occurs when there is increased production of thyroid hormones, T4 and T3, in the body. The thyroid gland primarily releases T4 into the circulation, which is then converted to the more biologically potent T3 in peripheral tissues by two enzymes: monodeiodinase type I (5'D-I) and monodeiodinase type II (5'D-II). Increases in T3 levels result in several physiological effects, such as increased myocardial contractility and faster diastolic relaxation of the heart and reduced systemic vascular resistance. These changes can increase the risk of developing high-output cardiac failure or even shock in patients, necessitating prompt and effective treatment.
Mechanism of action of Propranolol in Thyrotoxicosis
Propranolol is a non-selective beta-1 and beta-2-blocker that can effectively address tachycardia and tremors associated with thyroid storm. Importantly, it also inhibits the monodeiodinase type I enzyme responsible for the conversion of T4 to T3. This action reduces the metabolism of T4 and causes it to be shunted through the enzyme monodeiodinase type III (5'D-III), producing the metabolically inactive 3,3',5'-triiodothyronine (reverse T3 or rT3).
Moreover, propranolol's beta-2-blocking properties can help counteract the reduced systemic vascular resistance observed in patients with thyrotoxicosis. This is because blocking beta-2-receptors in blood vessels can result in vasoconstriction, thereby increasing systemic vascular resistance. Additionally, propranolol is a beta-blocker without intrinsic sympathomimetic activity, meaning it does not mimic the symptoms of thyrotoxicosis. Due to these multiple beneficial effects, propranolol has emerged as the most studied and commonly used beta-blocker in the management of thyrotoxicosis and thyroid storm.
Beta blockers, like propranolol, work by blocking beta-adrenergic receptors, which are present in various tissues and organs, including the heart, blood vessels, and lungs. By blocking these receptors, beta blockers interfere with the binding of catecholamines (epinephrine and norepinephrine) to these receptors. Catecholamines are hormones that mediate the "fight or flight" response and can cause an increased heart rate, blood pressure, and other symptoms. In thyroid storm or severe Graves' disease, the body produces excessive thyroid hormones, which can lead to increased catecholamine sensitivity and an overactive sympathetic nerv-ous system.
| Cardioselective | Non Cardioselective | |
|---|---|---|
| ISA present | Acebutalol | Pindolol |
| ISA absent | Atenolol, Metoprolol, Bisoprolol | Labetalol, Carvedilol, Propranolol |
Comparison To Other Beta-Blockers
Several other beta-blockers have been used in the treatment of thyrotoxicosis, but propranolol remains the most preferred option due to its unique properties. Its ability to inhibit the peripheral conversion of T4 to T3 and its beta-1 and beta-2-blocking effects sets it apart from other beta-blockers. These multiple actions provide a comprehensive approach to managing the various symptoms and complications associated with thyrotoxicosis.
Interestingly, the American Association of Clinical Endocrinologists Medical Guidelines for the Evaluation and Treatment of Hyperthyroidism and Hypothyroidism do not specifically recommend one beta-blocker over another when discussing the use of beta-blockers in this setting. Indeed, metoprolol is cited in the guideline as a suitable alternative to propranolol. It is worth noting that metoprolol, just like propranolol has no intrinsic sympathomimetic activity. Nonetheless, the preponderance of evidence supporting the use of propranolol in the management of thyrotoxicosis and thyroid storm suggests that it should be the preferred choice in most clinical scenarios.
Alternatives And Contraindications To Propranolol
In some cases, patients may have contraindications to the use of propranolol, such as asthma or reactive airway disease. In these instances, alternative medications like diltiazem, a calcium channel blocker, can be considered for the management of thyrotoxicosis. Diltiazem is known to have a similar effect on heart rate control, although it lacks the peripheral T4 to T3 conversion inhibition provided by propranolol.
Patients with concurrent low-output heart failure during thyrotoxicosis should be managed with caution when using negative inotropic medications, including propranolol. In these situations, careful monitoring and dose adjustments may be necessary to avoid exacerbating heart failure symptoms while still effectively managing thyrotoxicosis.
Dosage Considerations
The appropriate dose of propranolol for managing thyrotoxicosis and thyroid storm may vary depending on the severity of symptoms and the patient's individual response to therapy. Propranolol can be administered by mouth or nasogastric tube 60 to 80mg every 4 hours with close monitoring of cardiac function. Alternatively, intravenous propranolol 0.5 to 1mg slowly over 10 minutes, followed by scheduled 1-2mg boluses every few hours. This should only be done in a monitored setting.
Conclusion
Propranolol has emerged as the preferred beta-blocker for managing thyrotoxicosis and thyroid storm due to its multiple beneficial effects, including controlling tachycardia and tremors, reversing reduced systemic vascular resistance, and inhibiting the peripheral conversion of T4 to T3. The current evidence strongly supports its use as the first-line treatment in most clinical scenarios, although alternatives such as diltiazem may be considered for patients with contraindications to propranolol.
Future research should continue to explore the comparative effectiveness of propranolol and other beta-blockers in managing thyrotoxicosis and thyroid storm. Additionally, efforts should be made to update treatment guidelines to reflect the current evidence better, ensuring that patients receive the most appropriate and effective care for their condition.
References
Kindly Let Us Know If This Was helpful? Thank You!


