The Tricarboxylic Acid (TCA) Cycle, also known as the citric acid cycle or the Krebs cycle, is a central component of cellular metabolism. This metabolic pathway, occurring within the mitochondrial matrix, is responsible for the oxidation of acetyl CoA derived from carbohydrates, fats, and proteins into carbon dioxide (CO2) and hydrogen atoms, ultimately yielding energy through the production of adenosine triphosphate (ATP). This article will delve into the intricacies of the TCA cycle, illuminating its mechanism, regulation, and significance in cellular energy production.
Mechanism of the TCA Cycle
The TCA cycle begins with acetyl CoA, a two-carbon molecule derived from the breakdown of glucose via glycolysis, fatty acid oxidation, or amino acid catabolism. This molecule then combines with oxaloacetate, a four-carbon compound, to form a six-carbon citrate molecule, initiating the cycle. The cycle comprises eight steps, each catalyzed by a specific enzyme:
- Citrate Synthase: Acetyl CoA and oxaloacetate combine to form citrate, in a reaction catalyzed by citrate synthase.
- Aconitase: Citrate is isomerized into isocitrate.
- Isocitrate Dehydrogenase: Isocitrate is oxidized to alpha-ketoglutarate, reducing NAD+ to NADH in the process. This is the first of the three steps in the TCA cycle that produce NADH.
- Alpha-Ketoglutarate Dehydrogenase: Alpha-ketoglutarate is decarboxylated and combined with CoA to form succinyl-CoA, reducing another molecule of NAD+ to NADH.
- Succinyl-CoA Synthetase: Succinyl-CoA is converted into succinate, with the energy released in this step being used to generate ATP (or GTP, in some organisms) from ADP and inorganic phosphate.
- Succinate Dehydrogenase: Succinate is oxidized to fumarate, with the electrons being passed to FAD, reducing it to FADH2.
- Fumarase: Fumarate is hydrated to form malate.
- Malate Dehydrogenase: Malate is oxidized to regenerate oxaloacetate, reducing NAD+ to NADH.
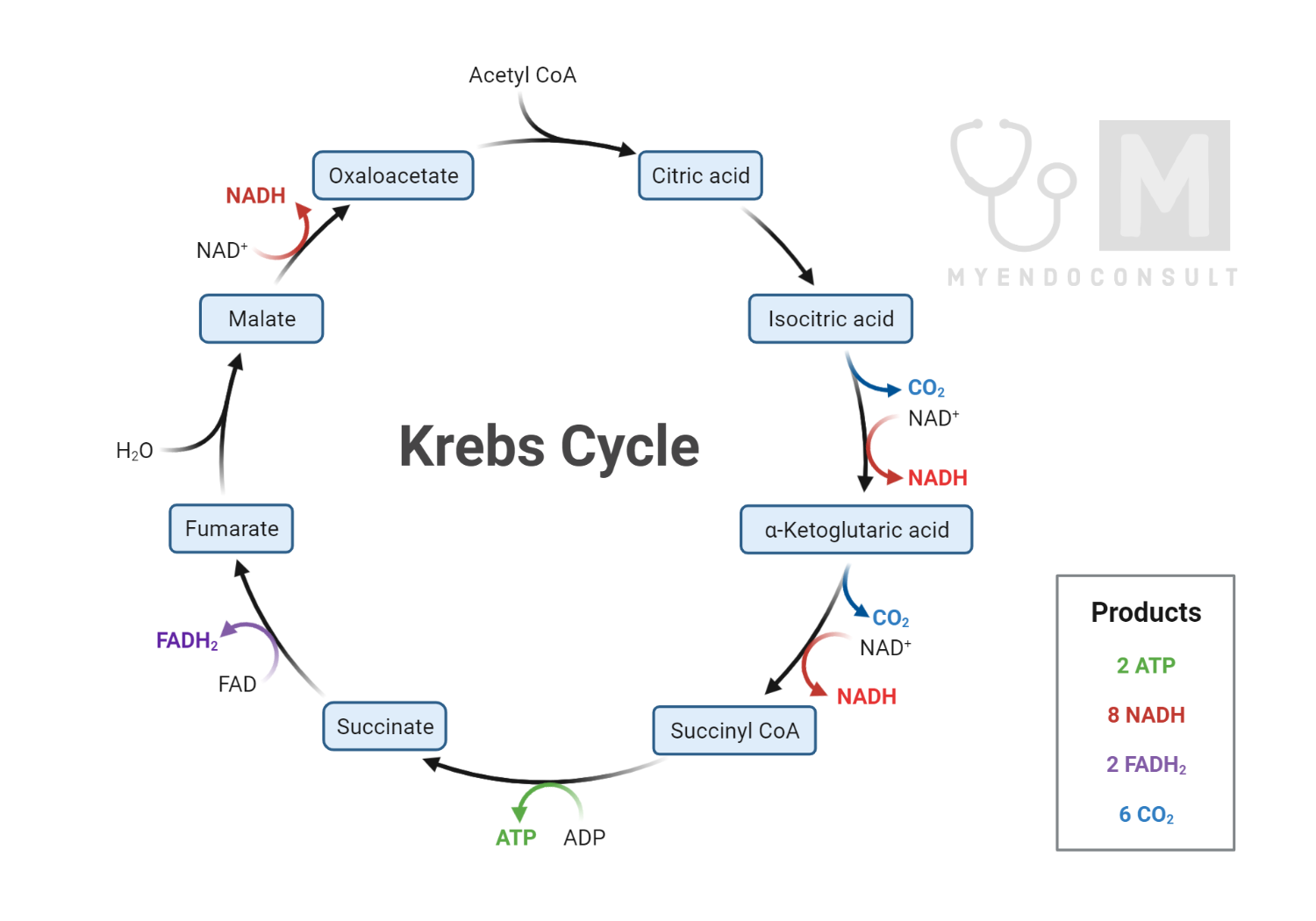
In total, one round of the TCA cycle generates three NADH, one FADH2, and one ATP, with the NADH and FADH2 molecules carrying electrons to the electron transport chain to further produce ATP.
Regulation of the TCA Cycle
The TCA cycle is tightly regulated to match the energy needs of the cell. Key enzymes, such as citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase, are controlled by allosteric effectors and product inhibition. For example, high levels of ATP and NADH, indicating a high energy state, inhibit these enzymes, slowing the TCA cycle. Conversely, a high ADP or NAD+ level, suggesting a low energy state, stimulates the cycle.
Importance of the TCA Cycle
The TCA cycle plays an indispensable role in cellular metabolism. It serves as a metabolic hub, intersecting with various pathways, including glycolysis, gluconeogenesis, lipid metabolism, and amino acid metabolism. As a primary energy-producing pathway, the TCA cycle is crucial for maintaining cellular energy homeostasis.
Warburg effect and the TCA cycle
The Warburg effect, named after Otto Warburg, who first described it in the 1920s, refers to the observation that cancer cells tend to favor anaerobic glycolysis for energy production even in the presence of ample oxygen. This phenomenon, also known as aerobic glycolysis, is unusual because it is less efficient in terms of ATP production than the typical process of oxidative phosphorylation that occurs in the mitochondria via the tricarboxylic acid (TCA) cycle and the electron transport chain.
Under normal physiological conditions, cells primarily generate energy (ATP) through oxidative phosphorylation, which includes the TCA cycle and the electron transport chain. Here, glucose is fully oxidized to carbon dioxide, with the electrons being transferred to oxygen via a series of carrier molecules, generating ATP in the process.
However, in the Warburg effect, the rate of glycolysis is significantly increased, and pyruvate, the product of glycolysis, is preferentially converted to lactate rather than being fully oxidized in the TCA cycle. This phenomenon happens even in the presence of sufficient oxygen that would usually allow pyruvate to enter the TCA cycle in the mitochondria.
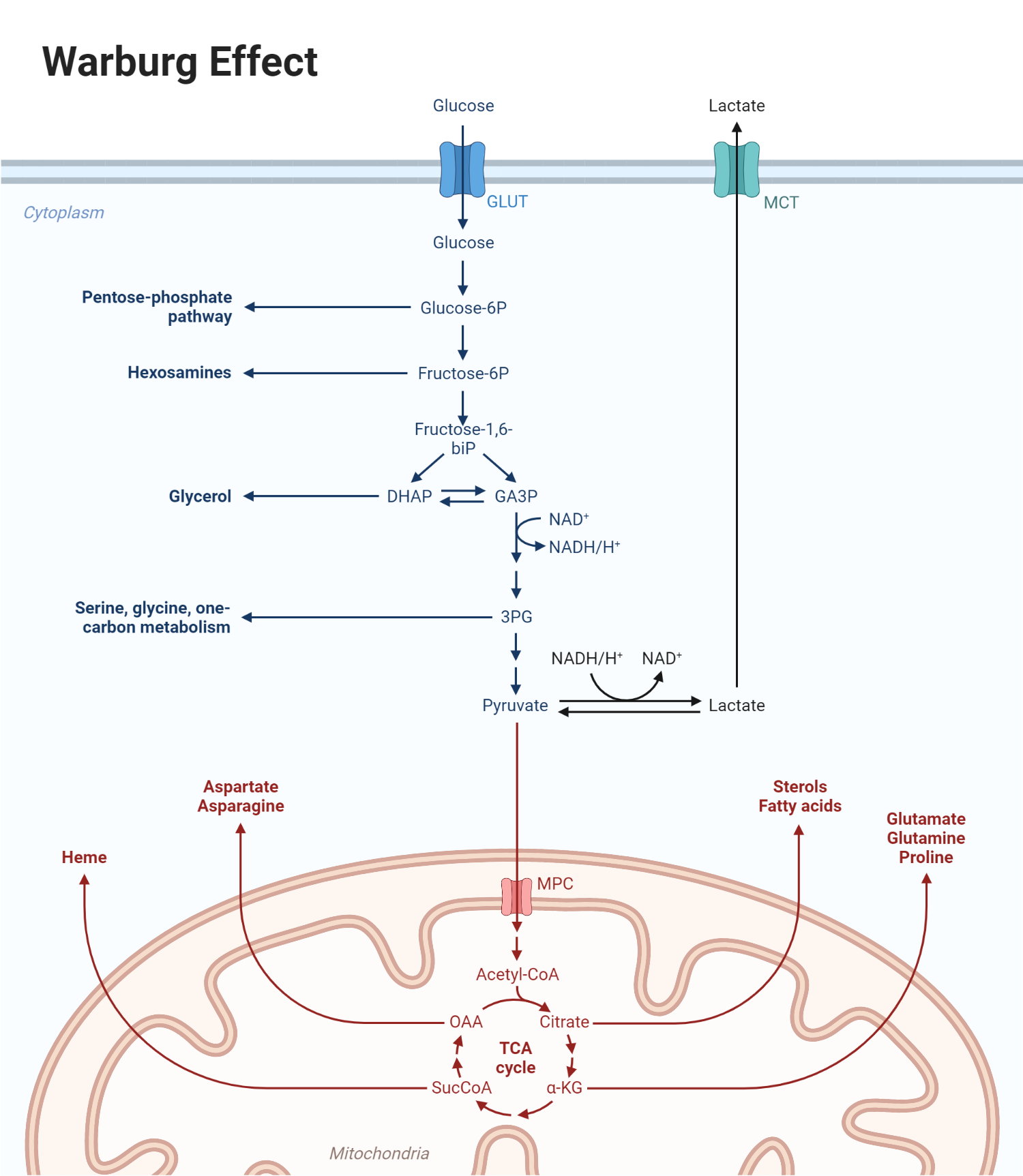
While the Warburg effect might seem counterintuitive given that glycolysis produces significantly less ATP than oxidative phosphorylation, it confers several advantages to rapidly proliferating cancer cells:
- Biosynthesis: The intermediates of glycolysis can be shunted to various biosynthetic pathways to produce nucleotides, amino acids, and lipids – essential building blocks for rapidly dividing cancer cells.
- Oxidative Stress Reduction: By relying on glycolysis, cancer cells can limit the amount of reactive oxygen species (ROS) produced by the electron transport chain, thereby reducing oxidative stress and potential damage to cellular components.
- Adaptation to Hypoxic Environment: As tumors grow, they often outstrip their blood supply, creating a hypoxic (low-oxygen) environment. By favoring glycolysis, cancer cells can continue to produce ATP even under low oxygen conditions.
Despite extensive research, the Warburg effect remains a topic of ongoing study. It is hoped that a better understanding of the metabolic alterations in cancer cells could lead to novel therapeutic strategies targeting the unique metabolic needs of these cells.
Kindly Let Us Know If This Was helpful? Thank You!



In the TCA diagram, I believe the NET production after 2 cycles should be (4) CO2, (6) NADH, (2) FADH2 and (2) ATP/GTP. I am unsure where the (6) CO2 and (8) NADH came from.