The effectiveness of hormone action is dependent on the hormone’s ability to bind to a receptor on the target cell. This binding process enables the cell to differentiate the hormone from other substances, subsequently triggering a cellular response. Additional regulatory control is exerted through the variation in the number of hormone receptors present on each type of target cell.
Hormones can be classified into two groups based on where their receptor is located on the target cell: either on the cell surface (cell surface receptors) or inside the cell at the nucleus level (nuclear receptors).
Peptide hormones predominantly bind to cell surface receptors, whereas amino acid derivatives and steroid hormones are typically ligands for nuclear receptors.
Notably, alterations in the structure of a hormone receptor due to mutations can cause the receptor to be constantly turned off or on. In the former case, this can lead to clinical situations of hormone deficiency, and in the latter case, it can cause hormone excess.
When a hormone arrives at its target cell, it first needs to identify and connect with it before influencing it through a process known as signal transduction. The initial step is the recognition between the hormone and the target cell. This interaction demands the presence of a particular molecule on the target cell that the hormone can bind to, known as the receptor.
This receptor can be positioned in various locations. It may be located in the plasma membrane of the target cell with an extracellular domain protruding beyond the membrane into the circulating medium. Alternatively, it might be situated inside the cell, typically in the cytoplasm or nucleus.
To date, hundreds of receptor proteins have been discovered. In an ideal scenario, a receptor would be specific to a single hormone molecule. However, in reality, a receptor often has the ability to bind with other molecules that share a similar chemical structure, also known as ligands. This capability resembles how a ‘specific’ globulin in the blood can bind with more than one chemically similar hormone.
Receptors for Protein and Polypeptide Hormones
Given that protein and polypeptide hormones are lipophobic, they typically cannot infiltrate target cell membranes without the help of specific transport systems. As such, these hormones usually have their receptors embedded in the plasma membranes of their target cells. Certain eicosanoids, like prostaglandins, also have cell surface receptors.
A standard receptor of this kind is a transmembrane protein consisting of an extracellular domain to which the hormone molecule can attach, a portion of the receptor stretching across the membrane, and an intracellular domain linked to a specific transduction process.
A well-known group of such receptors is the seven-transmembrane receptor family, named so because every receptor in this group possesses seven membrane-spanning helices that connect the extracellular and intracellular domains. The intracellular domain is connected to a specific intracellular guanine nucleotide-binding protein, known as a G protein.
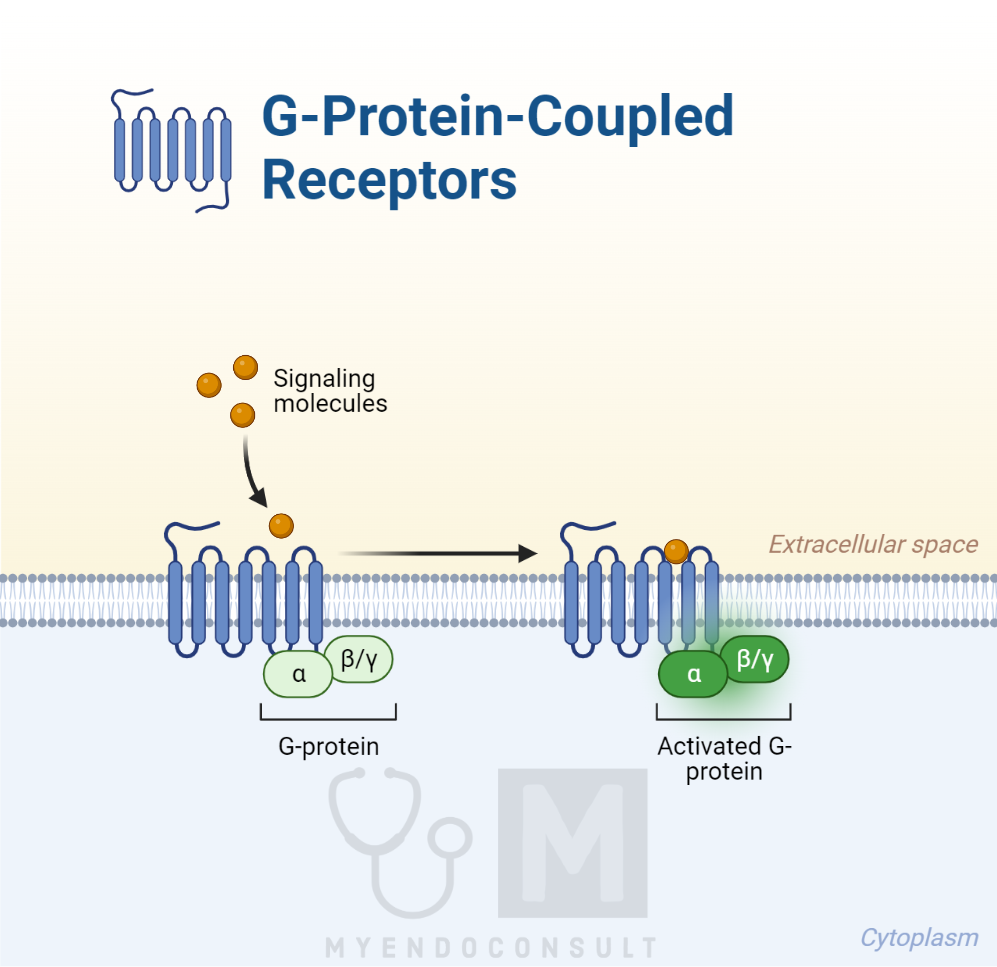
This G protein is the first part of the intracellular transducing mechanism that activates the cell in a particular way. Therefore, these receptors are termed G protein-coupled receptors (GPCRs). GPCRs are regulated by complex mechanisms, with at least 30 intracellular molecules, known as regulators of G protein signaling, identified so far that can influence them. Other unique intracellular molecules that can activate the G proteins, called activators of G protein signalling (AGS), have also been identified. The intricate regulation of G proteins within the cell adds an extra layer of complexity to the cell’s response to hormone-induced processes.
Certain membrane receptors might consist of a single strand spanning the membrane, featuring an extracellular domain with hormone-binding capability, and an intracellular domain possessing inherent enzyme activity when activated. Such receptors are classified by the enzymes found on their intracellular domains, like tyrosine kinase receptors, for example.
A variation of this receptor is the homodimer, which essentially is a double single-spanning receptor. There is also evidence to suggest the existence of heterodimers, where two different receptors can link together. These receptors can be single-spanning membrane receptors or GPCRs, indicating some exciting potential, particularly when each receptor is linked to different intracellular second messenger systems. These second messengers are the molecules that relay the hormone’s response within the cell.
When the hormone or other ligand binds to the extracellular domain, the receptor undergoes a conformational change, resulting in the activation of the enzyme linked to the intracellular domain or G protein. The activation of this intracellular enzyme is associated with the transduction of the hormone’s ‘message’ to the interior of the target cell.
Ligand-gated channels
As mentioned previously, hormone molecules that are released into the bloodstream serve as primary messengers, carrying information from one part of the body to another to initiate a specific reaction. Generally, when a hormone attaches to its receptor, it can trigger the activation of particular pathways that involve other intracellular secondary messenger molecules. For proteins, polypeptides, and other hormones that connect to the extracellular binding sites of their receptors in the target cells’ plasma membranes, various secondary messengers can be produced.

Each of these is connected with one or more specific intracellular pathways. These pathways typically consist of chains of activated enzymes that lead to the generation of the correct response to the initial trigger, which can range from the opening of an ion channel to the creation of a new protein molecule.
The mechanisms of action partially depend on the types of membrane receptors that the hormones connect to. The two primary receptor types relevant to most hormones are enzyme-coupled receptors and the more prevalent G protein–coupled receptors.
Certain receptors are integrated into the molecular architecture of ion channels in the plasma membrane. These are frequently termed ligand-gated channels to differentiate them from other types of ion channels, such as voltage-gated and stretch-activated channels. Notable examples include neurotransmitters like acetylcholine and gamma-aminobutyric acid (GABA), which are crucial for nerve impulse transmission in the nervous system.
When the messenger molecule (the ligand) attaches to the extracellular part of the receptor, which is a part of the ion channel, it can trigger the opening of the channel to specific ions. This allows ions to move along their electrochemical gradients across the cell membrane. This ion movement can then change the electrical properties of the cell, which can lead to further cellular responses such as the generation of an electrical signal in neurons. The rapid response of these receptors makes them ideal for processes that require immediate reaction, like synaptic transmission in the nervous system. Thus, ligand-gated ion channels play a fundamental role in translating extracellular signals into cellular responses.
Enzyme coupled receptors
Enzyme-coupled receptors in the cell membrane offer a unique mechanism where the detection of the target cell is combined with a direct, specific response. This class of receptors involves the presence of an intracellular enzyme as part of the receptor’s structure. Consequently, when a hormone or ligand binds to this type of receptor, it activates the internal enzyme. Some known examples are receptor tyrosine kinases, receptor serine and threonine kinases, and receptor guanyl cyclases.
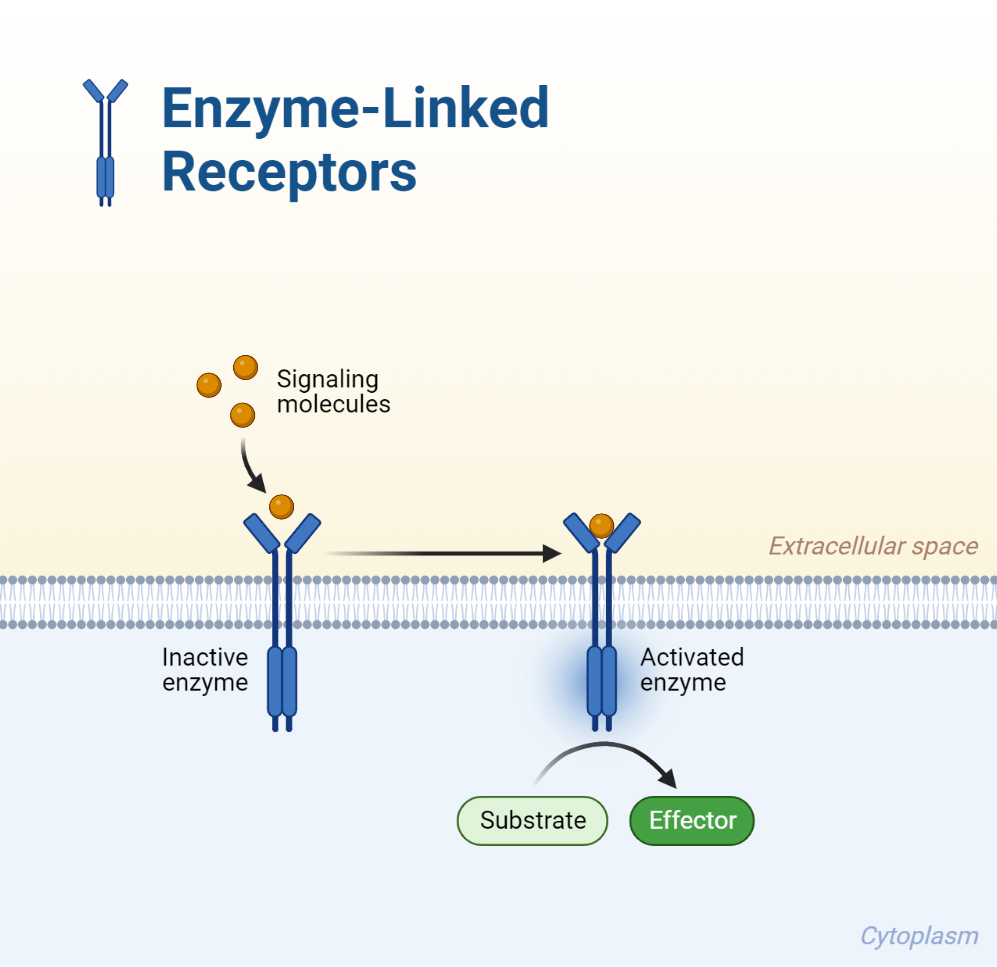
When the ligand attaches to the extracellular binding site on the receptor, which spans the cell membrane, it sparks the intracellular enzyme component into action. This enzyme is then capable of modifying specific amino acids in associated proteins through a process called phosphorylation. Some of these receptors are single-strand transmembrane proteins, while others can pair with neighboring receptor molecules to form homo- or heterodimers.
Take the case of growth factors activating tyrosine kinase-coupled receptors, leading to the formation of homodimers, where two identical receptors join together. This activation leads to the tyrosine kinases phosphorylating tyrosyl residues in the intracellular components of the receptors. This can result in autophosphorylation, which extends and amplifies the activity related to the activation of the receptors, and/or activate tyrosyl residues in other intracellular proteins.
There are numerous intracellular proteins that can be activated by phosphorylation. Thus, the activation of different intracellular ‘second messenger’ pathways can occur, involving a variety of intracellular proteins. This can result in more than one biological response to the initial hormonal signal. Furthermore, the activation of a single receptor kinase can lead to the phosphorylation of a sequence of intracellular proteins, creating an internal amplification of the initial hormonal stimulus. This process is essential in the action of hormones such as insulin and various growth factors, which bind to tyrosine kinase receptors.
G protein-coupled Receptors (Detailed Review)
G protein-coupled receptors (GPCRs) represent a vast group of transmembrane receptors that share a similar structure. This structure includes an extracellular domain for ligand (hormone) binding, seven membrane-spanning loops, and an intracellular enzyme-linked region that activates upon ligand binding. A range of hormones interact with this group of receptors, including adrenaline, glucagon, vasopressin, and parathormone.
The intracellular region of a GPCR houses a guanine nucleotide protein, composed of three subunits named α, β, and γ. Both α and γ subunits are attached to the cell membrane. In the receptor’s resting state, the Gα subunit is inhibited by the Gβγ subunits and linked to a guanosine diphosphate (GDP) molecule. When a hormone binds to the extracellular domain of its receptor, it triggers a structural shift in the receptor, activating the Gα subunit. This subunit then favors the binding of intracellular guanosine triphosphate (GTP) over GDP. This GTP binding overpowers the inhibitory Gβγ subunits, causing their detachment.
The activated Gα–GTP stimulates a specific intracellular enzyme, initiating the formation of a second messenger. This secondary messenger then sets off a chain of intracellular events, culminating in the appropriate cellular response to the initial hormonal trigger. The GTPase activity of the α subunit then hydrolyzes GTP back to GDP, resetting the G protein to its initial state. The various Gα subunits can either stimulate or inhibit certain membrane-bound enzymes, for instance, adenyl cyclase.
Adenyl cyclase is a key intracellular enzyme that forms part of the primary intracellular second messenger systems. When activated, it converts adenosine triphosphate (ATP) into the intracellular second messenger cyclic adenosine monophosphate (cAMP), releasing two phosphates. There are 10 known isomers of adenyl cyclase, each initiating specific cAMP-activated pathways. A crucial intracellular protein activated by cAMP is PKA, which phosphorylates other intracellular proteins.
Another membrane enzyme, phospholipase C, is embedded in the plasma cell membrane and catalyzes the formation of an important intracellular second messenger system upon activation. It breaks down a membrane phospholipid, phosphatidylinositol-4,5-bisphosphate (PIP2), into two components, inositol triphosphate (IP3) and diacylglycerol (DAG), which serve as secondary messengers. These messengers then activate a sequence of intracellular events.
Intracellular events that raise the free calcium ion concentration within the cell through second messenger-induced mechanisms can stimulate various subsequent reactions. As calcium ions are pivotal in activating numerous intracellular chemical reactions, regulating their intracellular concentration often plays a critical role in controlling a cell’s response to stimuli. Calcium ion channels located in the plasma cell membrane or within internal organelles like the endoplasmic reticulum can regulate the cytoplasmic free calcium ion concentration. Hormones can directly influence these calcium ion channels or do so indirectly through the formation of second messenger transduction pathways. The opening of cell membrane calcium channels results in calcium ions flowing into the cytoplasm, temporarily increasing the intracellular concentration due to the significantly lower intracellular concentration of calcium ions compared to extracellular concentration.
Similarly, the opening of calcium channels in the membranes of internal organelles, such as the sarcoplasmic or endoplasmic reticulum, results in the migration of these ions from the high-concentration storage sites within these organelles to the cytoplasm. Ordinarily, the concentration of free calcium ions in the cytoplasm is quite low (around 0.1μM). Consequently, any rise in its concentration or the frequency of its fluctuations can greatly influence signaling processes within the cell. Specifically, calcium ions can bind to a protein called calmodulin, triggering the activation of calmodulin-dependent kinases. These kinases have the fascinating ability to phosphorylate the cAMP response element binding protein (CREB), a critical factor in DNA transcription.
Thus, a ligand, such as a hormone, can attach to its GPCR, which subsequently regulates various cytoplasmic events. These include direct control of various enzymes and manipulation of the intracellular free calcium ion concentration. The impacts of these activities tend to emerge rapidly, within seconds to minutes. Yet, that same ligand might also steer longer-term events, lasting hours or even days, such as the creation of new proteins. These new proteins could be fresh intracellular enzymes or peptides and proteins intended for export from the cell.
In a clear illustration, cAMP molecules cause the separation of inhibitory regulatory units attached to protein kinase A (PKA), thereby activating the PKA molecules. These molecules then cross the nuclear membrane through nuclear pores, contributing to the intricate cellular response network.
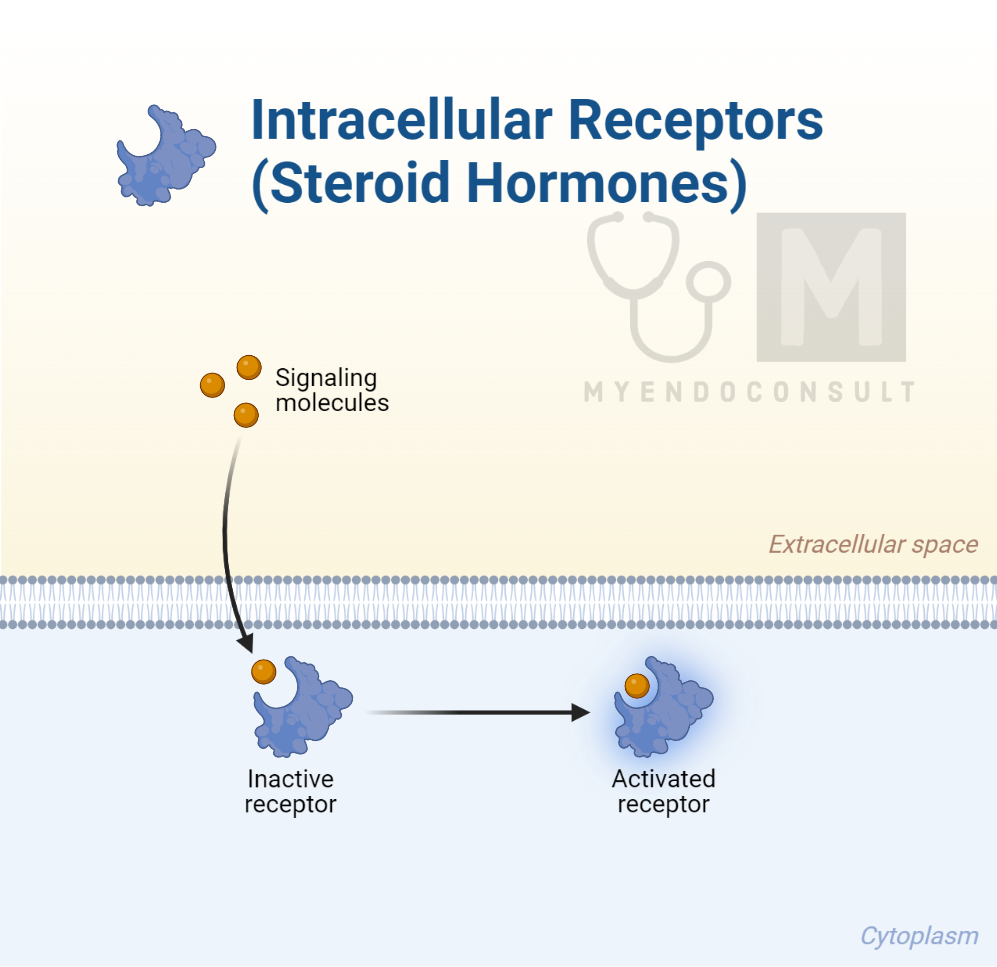
Steroid hormone receptors
Steroid hormones exert their influence by interacting with intracellular hormone receptor complexes, which serve as transcription factors. These factors attach themselves to specific areas on the target chromosome, typically proteins like histones, that function as DNA-binding sites known as promoters. It’s well established that chromosomes display a bead-like appearance due to the presence of nucleosomes, which are regions of tightly coiled DNA wrapped around protein structures, including histones.
The DNA segment that contains the genetic instructions to be transcribed into mRNA for the final protein product is known as the transcription unit. It comprises introns and exons, but only the exons undergo transcription. Furthermore, there is a promoter or regulatory region situated up-stream of the transcription unit on the 5′ end of the DNA strand. The transcription factor, in this case, the hormone-receptor complex, will bind to this region. Additionally, there’s a specific transcription termination site that marks the endpoint of that particular gene transcription.
As previously discussed, the transcribed mRNA is ultimately translated into a new protein, which facilitates the cellular response to the hormone. Interestingly, lipophobic hormones such as proteins and polypeptides can indirectly influence genomic activities.
Kindly Let Us Know If This Was helpful? Thank You!


