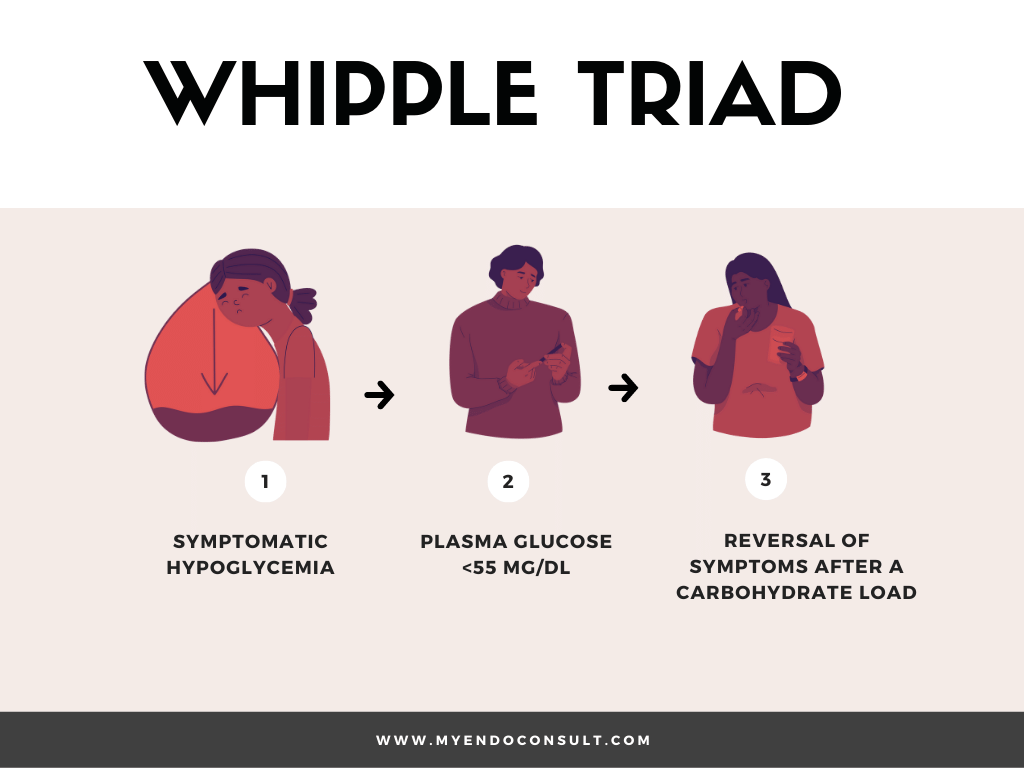
Whipple triad is defined as symptomatic hypoglycemia, biochemically confirmed hypoglycemia (plasma glucose <55 mg/dl), and reversal of hypoglycemic symptomatology after correction of hypoglycemia.
Introduction
The classic Whipple triad was first described by Allen Oldfather Whipple in a paper titled “the surgical therapy of hyperinsulinism” in 1938. Neuroglycopenic symptoms of hypoglycemia include confusion, unusual behavior, seizures and visual changes. The sympathoadrenal symptoms of hypoglycemia are tremulousness, diaphoresis and palpitations.
What are the elements of Whipple’s triad?
The triad described in patients with true hypoglycemia is termed the Whipple triad and is primarily composed of (1) hypoglycemia symptoms, (2) hypoglycemia (blood glucose levels < 55 mg / dL), and (3) relief of symptoms after ingesting glucose).
Working definitions of hypoglycemia
Severe hypoglycemia : Symptoms of hypoglycemia requiring assistance from another person (in the form of a glucose load, glucagon or other resuscitative actions).
Symptomatic hypoglycemia : Typical symptoms of hypoglycemia with a measured plasma glucose of less than 70mg/dl (3.9 mmol/l).
Asymptomatic hypoglycemia : Measured plasma glucose of less than 70mg/dl (3.9mmol/l) in the absence of the typical symptoms of hypoglycemia.
Relative hypoglycemia : Typical symptoms of hypoglycemia with a measured plasma glucose greater than 70mg/dl (3.9 mmol/l)
Pathophysiology
The brain needs glucose as fuel under normal physiological conditions. For the proper functioning of the brain, plasma glucose should remain in an essentially normal range. Several counterregulatory measures to prevent or treat hypoglycemia are at play under normal conditions.
The main protections against hypoglycemia are: 1) Decrease in insulin secretions, 2) Increased glucagon levels 3) Increased epinephrine production and finally an 4) increase in cortisol. In case of failure of such measures, the plasma glucose level is likely to decrease further.
Physiological responses to hypoglycemia
Under normal physiological conditions, there is a balance between glucose utilization and glucose production. When glucose utilization exceeds glucose production, hypoglycemia occurs.
Glucose utilization can be attributed largely to the brain, obligatory glycolytic tissues such as the renal medulla, red blood cells and skeletal muscle. Conversely, the sources of glucose production include oral carbohydrate intake, kidney and the liver. The physiological responses to hypoglycemia can be grouped into four critical stages.
- Stage I : A decrease in insulin production by beta cells of the pancreas.
- Stage II : An increase in glucagon production by alpha cells of the pancreas
- Stage III : An increase in epinephrine production by the adrenal cortex just when glucose declines below the physiological range
- Stage IV : An increase in cortisol and growth hormone production after a period of sustained hypoglycemia.
It is worth noting that behavioral responses to hypoglycemia (extreme hunger) typically occur at a plasma glucose concentration at or below the 55mg/dl (3.0 mmol/l) threshold.
A significant reduction in insulin production occurs at this threshold (Stage I). Plasma insulin levels fall below 3u/ml (18 pmol/L), C-peptide below 0.6ng/ml (0.2 nmol/liter) and proinsulin levels below 5.0 pmol/L.
Causes of hypoglycemia in adults
Medications
- Insulin
- Insulin secretagogues (sulfonylureas, meglitinides)
- Alcohol
- Quinine
- Artesunate/artemisinin/artemether (anti-malarial agents)
Acute critical illness
- Organ failure (hepatic, cardiac or renal)
- Severe septicemia
Loss of counter-regulatory hormone production
- Cortisol (adrenal insufficiency)
- Glucagon and epinephrine (type 1 diabetes mellitus)
Endogenous hyperinsulinemia
- Insulinoma
- Nesidioblastosis also known as functional beta cell disorders. These include post gastric bypass hypoglycemia and noninsulinoma pancreatogenous hypoglycemia.
- Autoimmune insulin-mediated hypoglycemia (antibody to insulin or the insulin receptor)
- Insulin secretagogue
Exogenous insulin
- Malicious intent of patients, accidental or surreptitious exposure.
Diagnosis of hyperinsulinemic hypoglycemia
All of the following criteria should be fulfilled prior to the diagnosis of hyperinsulinemic hypoglycemia.
- Fulfillment of Whipple’s triad
- Elevated plasma insulin level of (> 3 μU/ml) and C-peptide (> 0.6 ng/ml)
- Negative sulfonylurea screen
Whipple triad versus the Warburg effect
| Whipple triad | Warburg effect |
| A diagnosis of true hypoglycemia with a plasma glucose <55mg/dl, hypoglycemic symptoms compatible with neuroglycopenia that are ameliorated by a carbohydrate load or glucagon. | A diagnosis of spontaneous asymptomatic hypoglycemia in nondiabetic patients which occurs as a result of avid glucose consumption by a solid tumor. The reliance of the brain on lactate (produced through the fermentation of glucose) results in an absence of the classic symptoms of hypoglycemia. |
References
- Liberti, Maria V, and Jason W Locasale. “The Warburg Effect: How Does it Benefit Cancer Cells?.” Trends in biochemical sciences vol. 41,3 (2016): 211-218.
- Goyal I, Ogbuah C, Chaudhuri A, Quinn T, Sharma R. Confirmed Hypoglycemia Without Whipple Triad: A Rare Case of Hyper-Warburgism. J Endocr Soc. 2020 Nov 21;5(1):bvaa182.
Images(s) Courtesy
MyEndoConsult
Kindly Let Us Know If This Was helpful? Thank You!


