In this article, we will review the embryology, gross anatomy and histology of the pancreas.
Historical timeline of discoveries in diabetes care
- Around 1500 B. C., Papyrus Ebers, Egypt; description of abnormal polyuria, possibly diabetes mellitus.
- 6th century B. C. The Indians differentiated the asthenic from the sthenic form of diabetes mellitus. In the Ayur Veda of Susruta, the illness is termed as “madhumeha” or “honey-urine”.
- A few centuries B. C., the Chinese recognized the sweet taste of the urine.
- 30 B. C. to 50 A. D. Aulus Cornelius Celsus described a condition in which much urine was excreted.
- 30 to 90 A. D. Aretaeus Of Cappadocia gave the same description as Celsus and called it “diabetes”.
- 131 to 201 A. D., Galen (an anatomist) described diabetes as a weakness of the kidneys.
- 860 to 932 Rhazes, an Arabian physician, discussed the treatment of diabetes mellitus
- 980 to 1027 Avicenna believed that the liver was particularly affected in diabetes and observed the connection between diabetes, furunculosis, and impotence.
- 1621 to 1675 Thomas Willis, in Oxford, distinguished diabetes mellitus from diabetes insipidus, and showed that the urinary sugar was increased in the former condition.
- 1682 Johann Conrad Brunner observed polyuria and polydipsia in a dog after removal of the pancreas.
- 1774 Robert Wyatt suspected the presence of a substance similar to sugar in the urine and blood. He obtained this substance by evaporating the urine.
- 1776 Dobson demonstrated a fermentable sugar in the urine and the sweet taste of the blood of diabetic patients.
- 1788 Thomas Cawley suspected a connection between diabetes and changes in the pancreas.
- 1796 Rollo recommended a low-calory diet in the treatment of diabetes and described the smell of acetone.
- 1815 Chevreul identified the sugar in diabetes as glucose.
- 1806-1886 Bouchardat utilized fermentation tests, the polarimeter and solutions of copper salts, for estimating sugar. He substituted fat and alcohol for carbohydrates, emphasized the value of green vegetables, a low-calorie diet, and much physical activity. He introduced days of fasting and the use of alkali, and discovered gluten bread.
- 1848 Hermann Von Fehling described the urine test which was later named after him.
- 1849 Claude Bernard discovered glycogen in the liver, and the “piqure”. He made quantitative estimations of the sugar in the blood.
- 1869 Paul Langerhans discovered the islet cells of the pancreas.
- 1882 Chauffard and Hanot described the combination of pigment-cirrhosis and diabetes as “bronze diabetes”.
- 1889 Von. Recklinghausen revealed the nature of the two pigments of “bronze diabetes”, and introduced the term hemochromatosis.
- 1889 O. Minkowski and J. Von Mering incidentally discovered that total pancreatectomy in a suitable experimental animal produces diabetes.
- 1891 Giulio Vassale ligated the excretory ducts of the pancreas, which led to the destruction of the acini, but not of the islet cells.
- 1892 O. Minkowski produced temporary disappearance of diabetes in dogs by subcutaneous implantation of the excised pancreas.
- 1893 Laguesse suspected that the islet cells formed a hormone.
- 1895 v. Noorden developed a technique of dietary therapy, stressed the formation of sugar from protein, and introduced the course of oats as a treatment.
- 1898~1962 E. P. Joslin untiringly improved the treatment of diabetes mellitus.
- 1906 Naunyn studied the metabolism in diabetes, particularly in diabetic acidosis. He emphasized the familial occurrence of the disease and the value of a just adequate nourishment in the prophylaxis and in the treatment of the metabolic disturbance.
- 1908 Zuelzer gained an alcoholic extract from the pancreas, which after being injected, produced shock – probably of hypoglycemic nature – causing the trial to be discontinued.
- 1909 De Meyer gave the name insulin to the still hypothetical hormone of the islet cells.
- 1913 F. M. Allen became famous for his hunger cures. He also contributed to the knowledge of carbohydrate metabolism.
- 1918 C. K. Watanabe produced hypoglycemia in the animal with an injection of guanidine.
- 1921 N. C. Paulesco in Rumania reported “pancreine”, i.e. a blood sugar-lowering extract from pancreas of dogs or cattle, which he had discovered during the First World War (1914-18).
- 1921 Frederick G. Banting and Charles H. Best discovered insulin. F. C. Mann and T. B. Magath showed that hepatectomy results in hypoglycemia.
- 1924 B. A. Houssay and Magenta noticed that hypophysectomy increases sensitivity to insulin.
- 1924 Seal Harris suspected hyperinsulinism as a cause of spontaneous hypoglycemia.
- 1926 E. Frank, M. Nothmann, and A. Wagner introduced biguanidines into the treatment of diabetes which, however, was abandoned in 1940. Abel succeeded in crystallizing insulin.
- 1927 Wilder, Allan, Power, And Robertson published the first case of organic hyperinsulinism.
- 1929 Howland, Campell, Maltby, and Robinson removed an islet-cell tumor and cured a case of hyperinsulinism for the first time.
- 1936 H. C. Hagedorn produced the first reliable insulin with prolonged action.
- 1937 F. G. Young discovered meta-pituitary diabetes. H. R. Jacobs observed alloxan-hyperglycemia.
- 1942 Guest pointed out hypokalemia during the treatment of diabetic acidosis.
- 1942 M. Janbon noticed the hypoglycemic action of one of the sulfonamides recommended for the treatment of typhoid fever.
- 1943 Dunn, Sheehan, and Macletchie discovered alloxan-diabetes.
- 1944 A. Louba Tieres explained the mode of action of certain hypoglycemic agents
- 1955 H. Franke and J. Fuchs observed hypoglycemia produced by another sulfonamide, and suggested that it should be used therapeutically in diabetes mellitus.
- 1955 F. Sanger discovered the structural formula of the insulin molecule.
- 1957 G. Unger introduced phenethyl biguanide into the treatment of diabetes.
- 1957 S. A. Berson and R. S. Yallow measured the insulin content of the plasma by radioimmunological
- methods.
- 1964 H. Zahn in Germany, Katsoyannis in U.S.A, and Niu Ching-I in China (1965), all independently succeeded in synthesizing insulin.
- 1967 D. F. Steiner and P. Oyer isolated proinsulin.
- 1969 Mrs. D. G. Hodgkin discovered the three-dimensional structure of pig insulin.
Embryology and Histology of the Pancreas
In the human embryo (measuring 3 to 4 mm in length), two entodermal outpocketings arise on opposite sides of the primitive duodenum. One of the epithelial buds grows out from the dorsal wall of the gut, just above the hepatic diverticulum; it forms the dorsal pancreas. The ventral pancreas, on the other hand, originates in the caudal angle between hepatic diverticulum and gut.
When the embryo is about 12 mm long, the two primordia meet, and when it is about 16 mm, they fuse to produce a joint organ. With the exception of the major parts of the head and the uncinate process, which are derivatives of the ventral bud, most of the mature gland is formed by the dorsal pancreas anlage. Both primordia are crossed by an axial longitudinal duct.
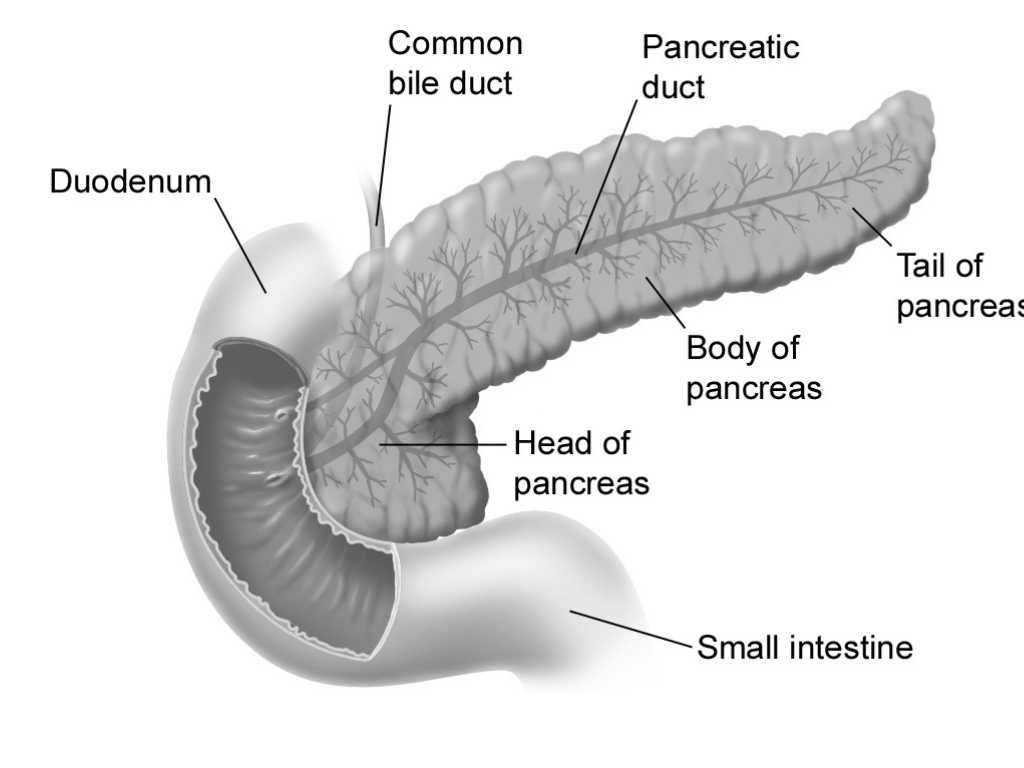
The duct of the dorsal anlage originates directly from the wall of the duodenum, whereas the ventral duct opens into the stem of the elongating common bile duct. When duodenal torsion has brought the two primordia into close side-by-side contact, the ventral duct taps its dorsal counterpart. The major pancreatic duct of the mature gland (duct of Wirsung) results, therefore, from the fusion of the ventral duct with the distal segment of the dorsal duct. The proximal stem segment of the dorsal duct, on the other hand, constitutes the accessory duct of Santorini, which usually retains its connection to the duodenum as well.
The islets of Langerhans develop from epithelial cells ofthe outgrowing pancreatic ducts. They are, therefore, of entodermal origin. Even in the embryo of 18 mm in length (age about 7 weeks), the terminal and side buds of the primitive ducts contain a few granular cells which can be selectively blackened by silver-salt solutions. These cells multiply and form single solid sprouts which enlarge to become the fetal (and early post-natal) islets.
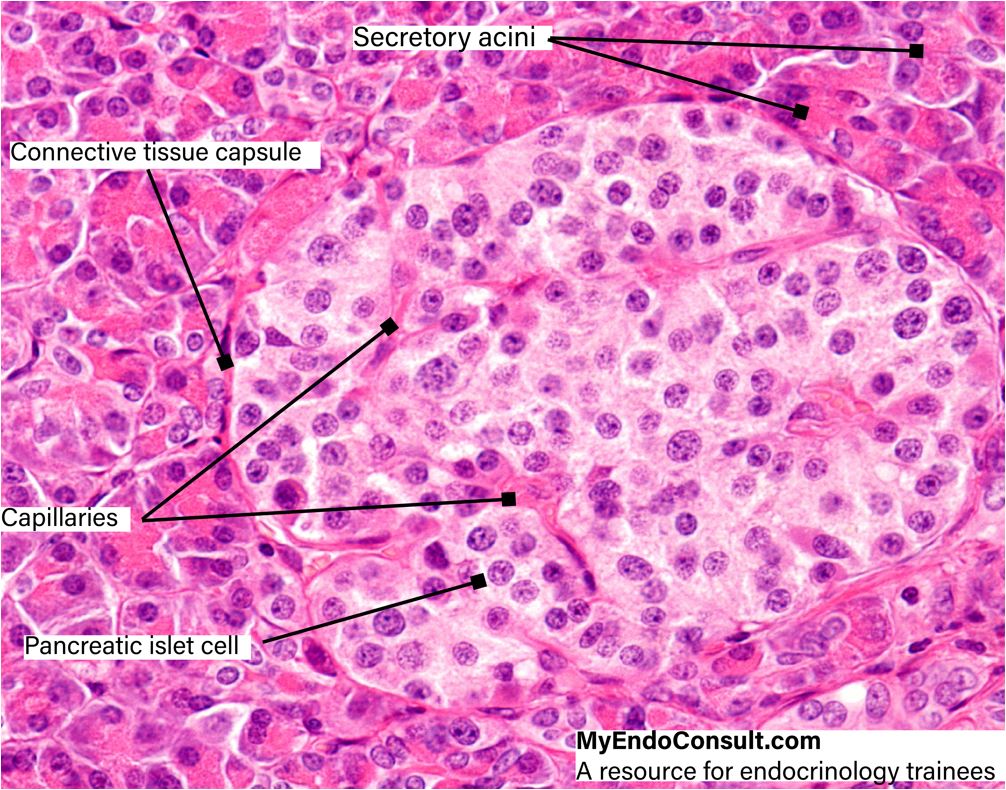
These so-called primary islets consist of a central mass of insulin-producing Beta cells which are surrounded by a compact layer of glucagon-synthesizing alpha cells. Between these two subdivisions, a transitional zone containing agranular cells soon develops. The core of the islet thus enlarges considerably and the islet itself resembles more and more the one found in the adult pancreas. The mature islets of Langerhans are rounded or ovoid epithelial complexes usually situated in the central portions of the lobules of the exocrine pancreas.
They may also, however, be found in the interlobular connective tissue or associated with the smaller branches of the pancreatic ducts. Although the islets are scattered throughout the exocrine pancreatic parenchyma, they are more numerous in the tail than in the head or body of the gland. The highly vascularized islets receive their blood supply through branches of the interlobular arteries which arise from both the celiac trunk and the superior mesenteric artery. The thin capsule of reticular fibers separating the islets from the exocrine parenchyma is usually penetrated by one arteriole. Within the islet, this arteriole splits up into a number of anastomosing capillary loops which are in intimate contact with the epithelial cords, like the vas afferens in a kidney glomerulum. The endothelium of these capillaries varies considerably in thickness. In attenuated portions of the endothelial cells, typical fenestrae (endothelial pores) are present.
Table . Pancreatic islet cells and their corresponding hormones
| Islet cell (frequency) | Hormone produced | Effects(s) |
|---|---|---|
| Beta cells (50-70%) | Insulin and amylin | Insulin increases peripheral glucose uptake and reduces hepatic gluconeogenesis and glycogenolysis. Amylin slows gastric emptying and stimulates satiety |
| Alpha cells (20-30%) | Glucagon | Stimulates hepatic gluconeogenesis and glycogenolysis. Stimulates hepatic ketogenesis during a prolonged fast |
| Delta cells (10%) | Somatostatin | Inhibits the secretion of insulin, glucagon, and PP |
| PP cells (2%) | Pancreatic polypeptide | Inhibition of glucagon secretion and acts as a satiety hormone |
| Epsilon cells (1%) | Ghrelin | Inhibits insulin release after a glucose load. Stimulates GH secretion and is also called the "hunger hormone." |
| G cells (absent) | Gastrin | Pancreatic gastrin-producing cells are present during embryonic development but undergo involution in adults. Re-expression of gastrin can, however, occur in the setting of pancreatic neuroendocrine tumorigenesis (Zollinger-Ellison Syndrome). |
| EC cells (rare) | Serotonin | Classic features of carcinoid syndrome |
Light microscopy reveals that the islet cells are less stainable than the acinar cells of the exocrine pancreas. In hematoxylin and eosin preparations, the cytoplasm of these cells appears more or less homogeneous, but with the Malloryazan stain, three types of cells can be easily distinguished, all of them displaying distinct granules in their cytoplasm. The alpha-cells, which are found predominantly at the periphery of the islets, contain granules which stain red and are insoluble in alcohol. The somewhat smaller granules of the more centrally located beta-cells, on the other hand, stain orange and are dissolved in alcohol. A third cell type, the so called delta-cell, is scattered throughout the islet. It displays granules which stain blue.
Electron microscopy shows that the alpha cells contain large numbers of membrane-bound, electron-dense granules. Characteristically, the granules are separated from their membrane by a very narrow, electron-translucent cleft. In human beta-cells, some of the granules contain rounded or polygonal crystalloid structures which display an internal periodicity.
The mitochondria of this cell type appear to be more numerous than in the alpha cells. The Golgi complex is well-developed. Between the granules, elongated and vesicular profiles of the rough-surfaced endoplasmic reticulum are intermingled with free ribosomes and small vesicles. The granules of the delta cells are larger and much less electron-dense than those of the alpha and beta cells.
References
Ferri V, Vicente E, Quijano Y, Ielpo B, Duran H, Diaz E, Fabra I, Caruso R. Diagnosis and treatment of pancreas divisum: A literature review. Hepatobiliary Pancreat Dis Int. 2019 Aug;18(4):332-336.
A-Kader HH, Ghishan FK. The Pancreas. Textbook of Clinical Pediatrics. 2012:1925–36.
Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7(5):473-9.

