
Brief history of glucose monitoring
Blood glucose is an important monitoring parameter when it comes to diabetes management. Attempts to quantify glucose in the urine date back to the mid-1800s, but the most important development in the commercialization of urine glucose testing was made in 1908 by Benedict, who developed a copper reagent for urine glucose.
The cumbersome methodology involved in glucose monitoring became more convenient in 1945 with the development of Clinitest, and in 1965 Ames developed the first blood glucose test strip, the Dextrostix, based on a glucose oxidase reaction.
By the mid-1970s, the concept of patients using blood glucose data at home was contemplated, and by 1980, the Dextrometer was launched. This meter used the Dextrostix along with a digital display. During the 1980s, meters and strips requiring less blood became available at a cheaper price. Self-monitoring of blood glucose (SMBG) became the standard of care, especially for patients with type 1 diabetes.
Through the late 1980s, 1990s, and early 2000s, SMBG technology continued to improve. The blood removal step was eliminated, smaller amounts of blood were required, electrochemical strips were developed, wider ranges of hematocrit were permitted, and new enzymatic tests were used.
The evolution of home glucose monitoring was further revolutionized with the introduction of continuous glucose monitoring (CGM). In 1999, the U.S. FDA approved the first “professional” CGM. The first “real-time” CGM was the Glucowatch Biographer, but it did not succeed.
In 2004, Medtronic introduced the Guardian REAL-Time CGM system, which could notify users of potentially dangerous hyperglycemia or hypoglycemia, and by 2006, the same company released the first integrated pump and sensor.
Dexcom and FreeStyle Navigator are followers of the Guardian, and all of them required blood glucose confirmation for treatment decisions to be made.
Modern times require modern solutions, so G4 Platinum in 2012, followed by G5 Mobile in 2015 allowed data to be transmitted to the user’s cellphone. The medtronic Minimed 630G integrated with thee Enlite sensor by Medtronic was the first insulin pump with “threshold suspends” for hypoglycemia. The threshold suspend featured allowed the pump to halt insulin infusion when anticipated blood glucose based on CGM readings was anticipated to be close to the limits hypoglycemia.
Medtronic’s first hybrid closed-loop device was available in 2017 using the Guardian Sensor 3. Abbott introduced the FreeStyle Libre Pro in 2016. This professional CGM was the first that required no fingerstick testing during wear. The professional CGM however could not be used for immediate treatment decisions by the patient. The data had to be downloaded at a later date for interpretation by a diabetes resource provider.
The FreeStyle Libre, for direct use by patients, became available in the United States in late 2017, allowing patients to read the data themselves. In less than 20 years, CGM has revolutionized the way diabetes is managed, especially type 1 diabetes.
Importance of glucose monitoring
Living with diabetes 24/7 is challenging. Understanding the value of continuous blood glucose monitoring can help patients with diabetes take charge of their self-management and play an active role in decision-making regarding their diabetes treatment.
Understanding their blood glucose monitoring data can motivate them to make healthier choices and build their self-confidence.
Advantages of Continuous Glucose Monitors (CGMs)
The advantages of CGM are many:
- It allows you to track your blood glucose levels any time of the day or night, understand what your blood sugar levels are doing at times when you don’t normally test, like when you are sleeping
- Share your data with your diabetes team remotely
- Get insight into how factors from your daily life including food, exercise, stress, medications, hormones, and illness affect you.
- Moreover, program alarms to alert you if your blood sugar is too high or too low, take action sooner to prevent dangerous hypos and tailor your insulin doses more carefully based on a better understanding of your blood sugar levels. These things can help overcome your fear of sudden hypos and have more confidence in your decision-making ability when administering insulin.
- Also, it reduces the number of finger-prick tests you need to do.
In the long term, analyzing your data with your diabetes team can help you come up with personalized strategies for your care and improve your HbA1c level over time through better day-to-day blood glucose management.
Understanding Continuous Glucose Monitors
What is a CGM?
Continuous glucose monitors (CGMs) constantly track your blood glucose levels, providing real-time updates via a device connected to your body.
Over the years, CGMs have gained popularity and become more precise, making them a reliable treatment choice for individuals with diabetes. A continuous glucose monitor (CGM) allows users to see if their glucose levels are trending high or low in real-time, enabling them to take preventive action against hypoglycemia and hyperglycemia. Real-time CGM monitoring has significantly improved outcomes for people with diabetes who, without a CGM, might have faced potentially life-threatening complications.
How does it work?
A CGM operates by using a small sensor inserted beneath your skin, typically on your abdomen or arm. This sensor measures your interstitial glucose levels, which refers to the glucose present in the fluid between cells. The sensor evaluates glucose levels every few minutes, and a transmitter wirelessly relays this information to a monitoring device.
This monitor could be integrated into an insulin pump or a separate device that you can carry in your pocket or bag. Some CGMs can also transmit data directly to a smartphone or tablet.
CGMs are constantly monitoring glucose levels during various activities, such as showering, working, exercising, or sleeping. Many CGMs come with special features that utilize data from your glucose readings, such as alarms that trigger when your glucose level is too high or too low. You can also record information about meals, physical activities, and medications within a CGM device.
Along with your glucose levels, you can transfer data to a computer or smart phone to analyze glucose trends. This is certainly helpful for your healthcare provider in assessing your diabetes control. Furthermore, certain models can instantly transmit information to a secondary person's smartphone, like a parent, partner, or caregiver. For instance, if a child's glucose level drops dangerously low during the night, the CGM can be configured to alert a parent in a nearby room.
The Dexcom series, Medtronic Guardian and Freestyle Libre sensors are approved for making treatment decisions. This means you can adjust your diabetes care plan based solely on CGM results. With legacy models from these CGM manufacturers, you had to first verify a CGM reading with a finger-stick blood glucose test before administering insulin or treating hypoglycemia.

Types of CGMs
- Dexcom G6 and Dexcom G7
- Freestyle libre, Freestyle libre 2 and Freestyle Libre 3
- Eversense senseonics
- Medtronic guardian sensor 3, and guardian sensor 4.
Many types of CGMs have evolved over the years, and there have been exciting improvements in the accuracy, reliability, and ease of use of these devices. Indeed, there are some specific differences between the two main types of CGMs, real-time and intermittently scanned. However, each has its unique feature that can impact its usefulness and acceptability in specific groups of patients.
Dexcom G6 : The Dexcom G6 CGM system sends real-time glucose readings automatically to a compatible smart device or Dexcom receiver. Fingersticks and scanning are not needed and even more importantly, it features a 10-day sensor easy to use.
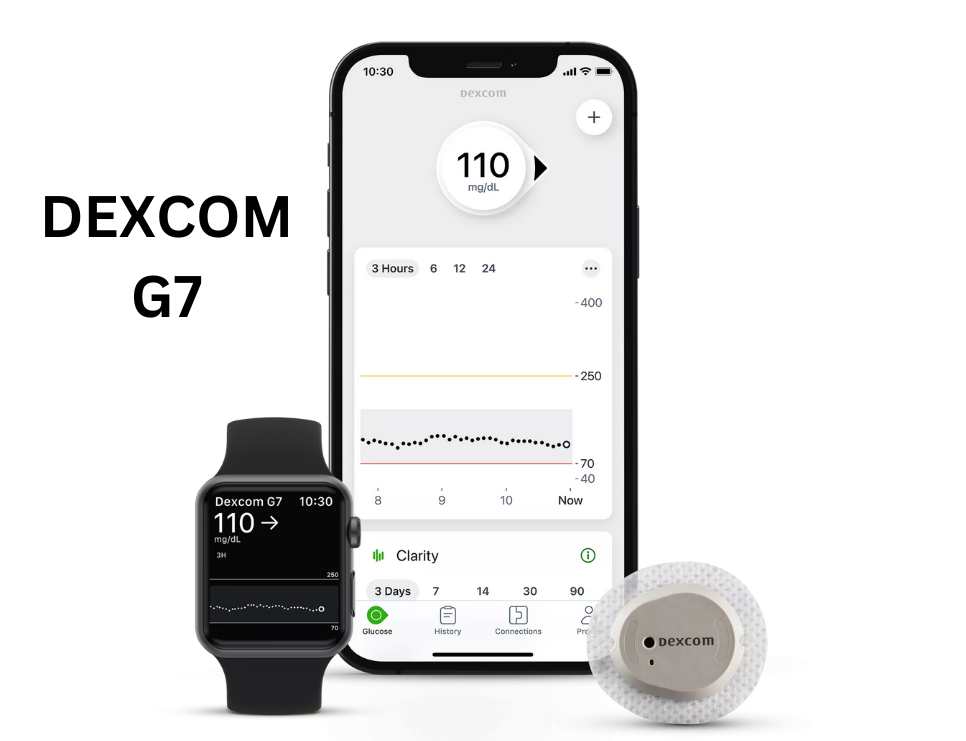
Dexcom G7 : Delivers real-time glucose numbers to a smartphone or a smartwatch. It also does not require fingersticks. The advantage of the G7 over the G6 includes it’s less bulky profile (60% smaller) and greater accuracy.

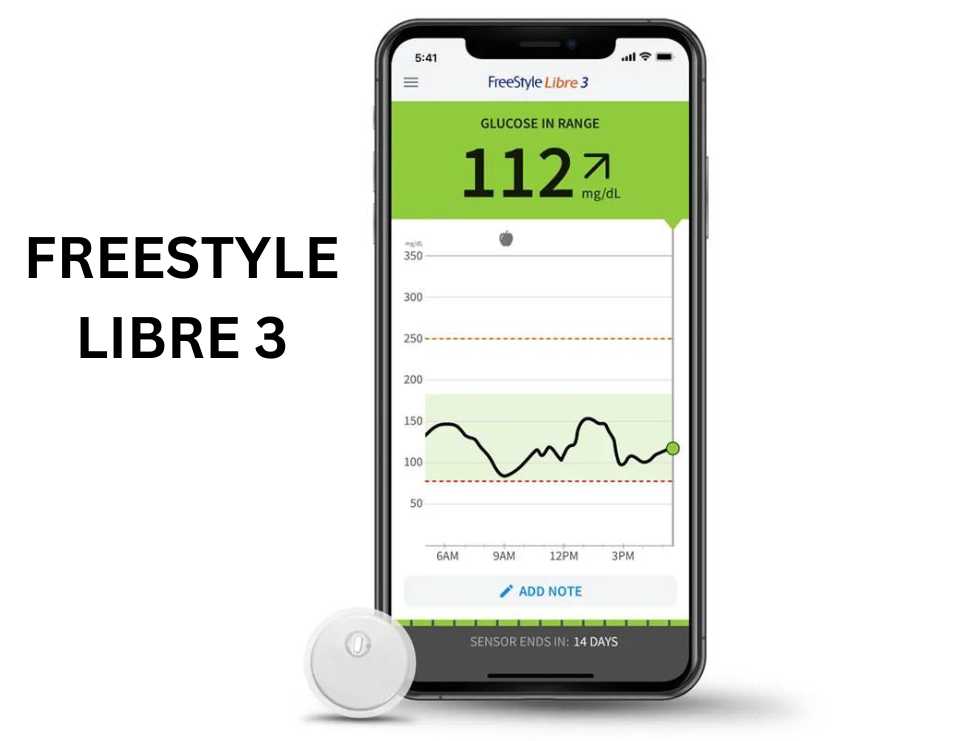
Freestyle libre 3 : The Freestyle Libre 3 is designed to be worn on the back of the upper arm and constantly monitors glucose levels in the interstitial fluid of the body, both day and night. The sensor refreshes glucose readings as often as every 5 minutes and directly transmits the data to a compatible smartphone. With a wear time of up to 14 days, the device is water-resistant, ensuring its functionality remains intact while swimming, showering, or exercising.

Eversense senseonics Although all CGM systems utilize sensors that are inserted through the skin and have a lifespan of 7-14 days, the Eversense sensor is uniquely implanted beneath the skin for long term glucose monitoring. With the Eversense CGM system lasting up to 6 months, it effectively eliminates the inconvenience and discomfort associated with frequent sensor insertions. Adoption of this sensor has however been limited for various reasons. The need for it’s insertion in a physician office may is a limitation. In addition, the need for fingerstick calibrations makes it less appealing to patients compared to the Dexcom G7 and Freestyle libre 3.
Medtronic guardian sensor 3 : This sensor has a Mean absolute relative difference (MARD) of 8.7%.
Medtronic guardian sensor 4 : The newest sensor from medtronic is the guardian sensor 4. It requires no finger sticks and also checks your blood glucose every five minutes. Medtronic’s state of the art hybrid closed loop insulin pump, the MiniMed™ 780G system, integrates with the guardian sensor 4 to proactively predict insulin requirements, fine-tune insulin delivery, and automatically correct high glucose levels while safeguarding against lows.
Accuracy and reliability
Most CGM systems assess glucose in the subcutaneous interstitial fluid, using the glucose oxidase approach. This provides minimal discomfort to patients and allows CGM usage at home.
Recent developments in CGM technology has improved their MARDs, making them very reliable and accurate enough to make treatment decisions.
Who can benefit from CGMs?
People with diabetes
Individuals with type 1 and type 2 diabetes on multiple daily doses of insulin (MDI) should utilize a CGM. The primary reasons for this preference among those with diabetes include increased feelings of safety, improved glucose management, reduced discomfort from finger sticks. Furthermore, the ability to connect with an insulin pump to create a closed-loop system (or artificial pancreas) helps to further optimize diabetes control.
Athletes and fitness enthusiasts
Keeping track of glucose levels can improve the quality of life for a fitness enthusiast. Having a positive impact on performance and energy levels is just one of the many benefits of glucose monitoring through a CGM.
For instance, endurance athletes may be training for long periods, so If they don’t fuel appropriately before and during training, they can risk low blood sugar – hypoglycemia. Hypoglycemia can lead to feeling a loss of energy, decreased performance, and even fainting.
Using the CGM, an athlete can monitor for dips in glucose or decreases in glucose during training and plan their fueling strategy accordingly. The CGM allows them to measure glucose during training without stopping and taking measurements. In addition, there is evidence that CGM devices are accurate during intense training. Although there is limited research, there is evidence that CGM can help increase performance and recovery.
Individuals with a history of hypoglycemia
A continued decline in glucose eventually leads to neurologic dysfunction, widespread electroencephalogram (EEG) changes, cognitive dysfunction, and severe neuroglycopenia. Counterregulatory abnormalities such as absent suppression of insulin secretion blunted or absent glucagon response, and blunted adrenaline response further exacerbate the hypoglycemic condition in Type 1 and advanced Type 2 diabetes. Patients who experience frequent episodes of hypoglycemia often develop these abnormalities and lose their ability to detect hypoglycemia; this is referred to as “hypoglycemia unawareness” and it perpetrates a vicious cycle of recurrent hypoglycemia. CGM can play a significant role in preventing hypoglycemia. Unlike traditional blood glucose monitoring, which looks at only a few points in time, CGM provides comprehensive data that track glucose levels 24 hours a day. This information allows patients to optimize their glycemic control and thus minimize the frequency and severity of hypoglycemic events.
Choosing a CGM
Factors to consider when choosing a CGM
Insurance coverage: Consult your insurance provider to determine coverage details, as some may restrict coverage to specific models
Cost: CGMs differ in price, so consider the cost of sensors since they will constitute the majority of the expense over time.
Ease of use: The usability of CGMs varies, with some requiring intermittent scanning or calibrations, making them less applicable to all patients with diabetes mellitus.
Comparison of popular CGMs in 2023
| Features | Dexcom G7 | Guardian Sensor 4 | Freestyle Libre 3 | Eversense E3 |
|---|---|---|---|---|
| Manufacturer | Dexcom | Medtronic | Abott | Senseonics |
| Mean Absolute Relative Difference | 8.20% | 9.60% | 7.90% | 8.50% |
| Approved Sites | Back of upper arms or upper buttocks | Back of upper arms or upper buttocks | Back of upper arms | Back of upper arms |
| Warm up | 30 minutes | 2 hours | 60 minute warm up | 24 hours |
| Duration of wear | 10 days | 7 days | 14 days | 180 days |
| Insulin pump integration | Tandem T Slim X2 (pending as of March 2023) | Minimed 780G | Not yet | Not yet |
| Smartphone app | Yes | Yes | Yes (not compatible with all models) | Yes |
| Sharing of data | Yes (Dexcom follow) | Yes (Carelink Connect) | Yes (libre link app) | Yes |
| Water Resistance | Yes | Yes | Yes | Yes |
Important Features of CGMS by brand
Different CGMs have different ways of use, but here are some examples:
Dexcom G5 Continuous Glucose Monitor:
• FDA-approved for non-adjunctive insulin dosing (treatment decisions) when blood glucose levels are between 80-250 mg/dL.
• Calibration is required twice daily when blood glucose is stable, preferably at home.
• If the sensor display lacks trend arrows, the CGM might be malfunctioning and should not be used for dosing.
• Acetaminophen (Tylenol) can falsely elevate CGM values; CGM data should not be used within 4-8 hours of acetaminophen intake.
Dexcom G6 Continuous Glucose Monitor:
• FDA-approved for non-adjunctive insulin dosing (treatment decisions) when blood glucose levels are between 80-250 mg/dL.
• Calibration is NOT required.
• Blood glucose must be checked via fingerstick if symptoms don't align with sensor readings.
• If the CGM reads "LO" or "HI," blood glucose should be checked with a fingerstick.
• If the sensor display lacks trend arrows, the CGM might be malfunctioning and should not be used for dosing.
• Acetaminophen does NOT affect the Dexcom G6.
Abbott Libre Flash Glucose Monitoring System:
• Users must scan the sensor with a device at least three times per day to obtain glucose data.
• Glucose readings appear on the meter display only AFTER the user scans the sensor with the actual meter.
Medtronic Guardian Connect CGM System:
• The Medtronic Guardian™ Sensor 4 is the newest CGM from medtronic. It requires no fingerstick calibrations.
CGMs and Time in Range
Time in Range (TIR) is more than just another diabetes metric; it is a tool that can be used to improve communication with your healthcare providers and empower you both in the doctor’s office and beyond.
Conversations about A1C with your healthcare providers can often feel one-sided. It can be difficult to discuss A1C results that you are seeing for the first time, leaving your healthcare team to do most of the talking. Because A1C is only an average measure of your glucose management over a period of three months, it cannot provide information about how your day-to-day actions affect your glucose. In contrast, Time in Range fosters a more dynamic conversation because people with diabetes can discuss the TIR data they live with day-to-day.
TIR is measured using a continuous glucose monitor), which measures glucose levels automatically every five minutes. These numbers can be graphed to show the percentage of time spent within a healthy glucose range between 70-180mg/dL (or, Time in Range), giving the user much more information about their glucose patterns each day.
A person with diabetes knows the most about their daily diabetes management, and TIR data gives them the tools to have engaging and productive conversations with their providers.
Insurance coverage limitations
Diabetes prevalence: One might assume that states with the highest diabetes prevalence would invest more in diabetes tools, but this is not always the case. Out of the 14 states where at least 12% of adults have diabetes, only 6 of them (AR, NM, IN, OH, TX, and WV) provide Medicaid coverage for CGMs for individuals with both type 1 and type 2 diabetes.
Medicaid spending: Among the top 10 states with the highest Medicaid spending (exceeding $15 billion in total), five do not cover CGMs for people with both type 1 and type 2 diabetes. This approach is not cost-effective, as CGMs represent only 1.1% of the total cost of diabetes, while expenses related to treating complications and lost productivity—both of which would be reduced through widespread CGM use—account for 73.1% of total diabetes costs. Moreover, using a CGM is cost-effective for individuals with diabetes.
Future developments in CGM technology.
In this modern world, everything makes a progress fast, so what are the expectations of the new technology?
PK Vitality, the company responsible for creating the K’Watch Glucose blood sugar monitor, has shared the outcomes of a recent clinical trial. The continuous glucose monitor (CGM) smartwatch demonstrated a high accuracy level. The device employs a combination of micro-points and biosensors to measure blood glucose levels without causing pain. In its first trial, the K’Watch Glucose CGM smartwatch had a Mean Absolute Relative Difference (MARD) of 29% for accuracy, which the developers managed to reduce to 19% in the second trial. The third trial yielded a MARD level of 16%, with modifications to the measuring algorithm and refinements to the K’apsul patch contributing to this promising accuracy level. What millions of people with diabetes, who are weary of using lancets and test strips, want to know is when the device will be available, its cost, and whether it will be covered by insurance.
References
Kindly Let Us Know If This Was helpful? Thank You!


