Adipose tissue is more than a simple inert tissue that acts as a fat storage. Adipose tissue is capable of accommodating increased lipids through hypertrophy of adipocytes and by initiating the differentiation of pre-adipocytes. Whole-body metabolism is impacted by adipose tissue metabolism. The endocrine function of adipose tissue includes synthesis and secretion of several hormones. These hormones play a vital role in a range of processes, which include the control of insulin sensitivity and inflammatory process mediators (TNF-α), control of nutrient intake (angiotensin, leptin), visfatin, resistin, adiponectin, and others. It’s endocrine function also covers control of acylation stimulating protein (ASP), plasminogen activator inhibitor 1 (PAI-1). Let’s review some of the endocrine aspects of adipose tissue and how it relates to inflammatory disease and insulin resistance.
Lipogenesis & lipolysis
Fat synthesis is known as lipogenesis, while fat breakdown is referred to as lipolysis or fatty acid oxidation. The balance between lipogenesis and lipolysis is what determines fat accumulation.
Lipogenesis occurs mainly in adipose tissue, but it may also occur in the liver. Lipogenesis is the synthesis of fatty acids which serve as energy reserves. Lipogenesis is responsive to dietary changes [1]. It is stimulated by a high carbohydate diet resulting in an increased postprandial plasma triglyceride level, whereas lipogenesis is inhibited by fasting and by polyunsaturated fatty acids. There is a strong correlation between fasting and a decrease in plasma glucose and elevation of plasma-free fatty acids. These effects are mediated by hormones in part, which stimulate or inhibit lipogenesis. Leptin inhibits lipogenesis, while angiotensin, and acylation stimulating protein stimulates lipogenesis. It is also worth mentioning that glucose is a substrate for lipogenesis. Glucose increases the process of lipogenesis by stimulating insulin release and inhibiting the secretion of glucagon from the pancreas [1].
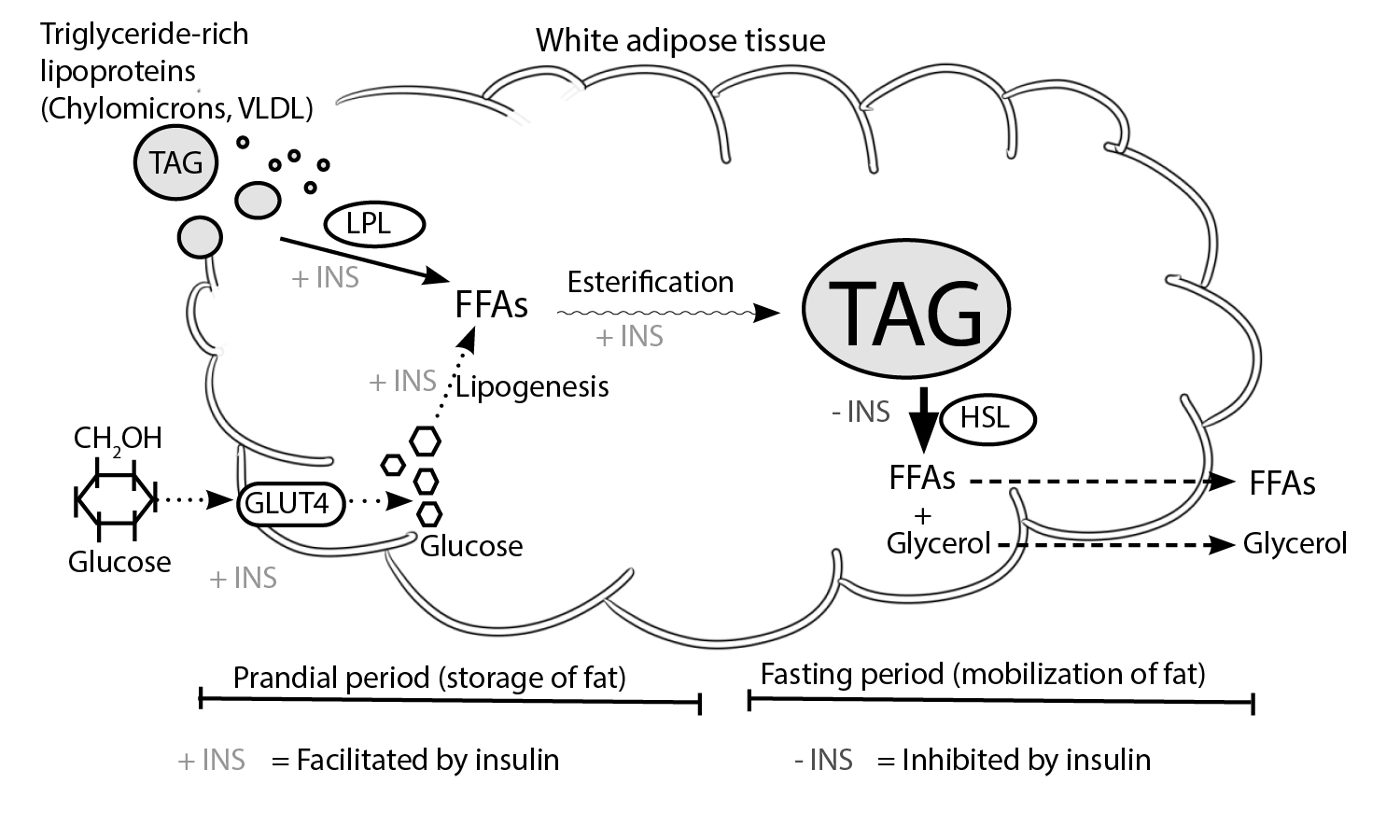
Lipolysis takes place in adipose tissue. It is characterized by the breakdown of fat for energy production. In lipolysis, molecules of triacylglycerol are hydrolyzed to form glycerol and free fatty acids. During metabolic stress (such as prolonged exercise or extended fasting), triacylglycerol droplets in the adipocytes are degraded to provide free fatty acid that may be used as a source of energy by other tissues. The lipolytic response in adipocytes may be elicited by numerous stimuli. However, monoacylglycerol lipase and hormone-sensitive lipase do catalyze the hydrolysis of the triacylglycerol ester bonds. Total hydrolysis of triacylglycerol involves splitting 3 ester bonds to release a glycerol moiety and free fatty acids. hormone-sensitive lipase facilitates the hydrolysis of esters at the first and third positions of the triacylglycerol. Another enzyme, 2-monoacylglycerol lipase catalyzes the hydrolysis of the third ester to form a third free fatty acid and glycerol. Insulin acts as inhibitor of hormone-sensitive lipase and is favored by the presence of epinephrine and glucagon [1, 2, 3]. Glycerol effluxes out of the adipocytes through a transport molecule and has to be shuttled back to the liver so it can be used for gluconeogenesis or in oxidation. It is important to note that under maximal lipolytic conditions, there is a substantial recycling of fatty acids resulting in the release of two fatty acid molecules per molecule of glycerol. Outside the adipocyte, the fatty acids bind to albumin and are transported in the general circulation to the muscle, liver, and other tissues for oxidation [2].
The free fatty acids that result from lipolysis undergo a catabolic process known as β-oxidation which allows them to serve as a source of energy to the body. The fatty acid molecules are converted into molecules of acetyl coenzyme A [4].
The secretory nature of adipose tissue
At first, researchers thought the adipose tissue to be a basic organ that served as a storage for lipid. Its primary function was believed to be mostly mechanical – where adipose tissue was thought to be a cushion that protected delicate organs from bumping and damaging. Adipose tissue was also believed to be the insulator that prevented the loss of heat from the body. Dr. GS Hotamisligil and BM Spiegelman made the first discovery that revealed that adipose tissue in obese and diabetic rodents inducted tumor necrosis factor-alpha (TNF-alpha) in these rodent models [5]. The researchers also observed RNA in adipose tissue from obese and diabetic rodents. Also, elevated protein levels of TNF-alpha was observed and the neutralization of TNF-α alleviated glucose intolerance in obese rodent models [5]. This was one of the earliest studies to indicate the secretory function of adipose tissues. A 1994 study by Friedman and colleagues provided the first evidence that adipose tissue is a major endocrine organ. This discovery came from the cloning of leptin [6]. The researchers found that fat cells secrete leptin into the blood and the leptin in turn acts on the brain to regulate intake of food and energy expenditure [7]. Prior to this, the identity of leptin had remained a mystery for four decades. Researchers however observed that there was a circulatory factor in ob/ob mice that triggered obesity. Several studies revealed that leptin is primarily a pleiotropic hormone whose functions extend wider than energy balance and appetite. Leptin acts as a signal molecule in immunity and reproduction [8, 9].
It is now recognized that adipose tissue secretes a diverse range of protein factors known as ‘adipokines.’ These adipokines play a major role in the maintenance of homeostasis and in the pathology of several diseases [10].
Adiponectin
The adiponectin gene is located on chromosome 3q27. It was first described in 1995. Looking at it structurally, it is important to note that adiponectin is related to the 11 complement family and contains an amino-terminal collagenous domain and a carboxyl-terminal globular domain. It shares a wide sequence homology with collagen VIII and collagen X [11]. There are three domains in each monomer of adiponectin namely a variable N-terminal region, a c-terminal globular domain that contains 140 amino acids, and a α-helical collagen composed of a number of G-X-X repeats [12]. There are three circulating isoforms of this adipokine: a trimer with a low molecular weight, a medium molecular weight hexamer, and a multimeric high molecular weight isoform [13]. Adiponectin is a full-length protein with a molecular weight of 30 kDa, circulating in trimeric, and hexameric complexes. Studies have shown that a fragment of adiponectin containing its globular domain exhibits strong metabolic effects in various tissues. The adipose tissue is the only tissue that secretes adiponectin [14]. There is a negative correlation between the concentration of adiponectin in human plasma and fat mass. The only exceptions are in the newborn and in severe cases of malnutrition [15]. Adiponectin is associated with type 2 diabetes, although this is mostly due to a decrease in the levels of high molecular weight isoform circulating in the blood. Adiponectin has been shown to improve whole-body insulin sensitivity, and stimulate fatty acid oxidation and glucose uptake in adipose tissue and skeletal muscle tissue. This effect, however, depends on AMP-activated protein kinase signaling. Adiponectin also plays a role in the suppression of hepatic glucose output through AMPK activation [12].
Interleukin-6
30% of interleukin-6 (IL-6) circulating in humans originates from adipose tissue. The concentrations of IL-6 are higher in visceral fat compared to subcutaneous fat. IL-6 concentrations increase with obesity and are stimulated by interleukin-1 (IL-1) and tumor necrosis factor (TNF). High levels are associated with elevated risks of atherosclerosis, coronary artery disease, and unstable angina [16].
The plasma levels of IL-6 are increased in type 2 diabetes and have a positive correlation with plasma free fatty acid concentrations and body mass. Cells of the stromal vascular fractions contribute the largest amount of IL-6, while about 1/3 of IL-6 found in the plasma comes from white adipose tissue [4, 12]. Studies have shown that IL-6 upregulates SOCS3 expression thus inhibiting the insulin signaling pathway. It is also important to note that the upregulation of SOCS3 expression impairs IRS-1 phosphorylation and insulin-induced insulin receptor in hepatocytes and adipocytes. IL-6 can promote the oxidation of fatty acids and glucose uptake in skeletal muscles [17]. Some studies show that these effects require the activation of AMPK-activated protein kinase [17]. In conclusion, IL-6 induces lipolysis, inhibits lipase lipoprotein, and increase uptake of glucose [12].
Angiotensin
All components of the renin-angiotensin-aldosterone system (RAA) are expressed by adipose tissue. These include renin, angiotensinogen, angiotensin I-converting enzyme and angiotensin II type 1 receptor [18]. The protein levels and mRNA in adipose tissue angiotensinogen are regulated by nutrition, resulting in decreased levels with fasting and high levels with refeeding. Lipogenesis, adipocyte differentiation, and prostacyclin synthesis are all stimulated by angiotensin II. Based on these findings, researchers suggest that adipose tissue-derived angiotensin may regulate the differentiation and growth of adipocyte. There is also the possibility that RAAS peptides act on distant targets and on the vasculature to regulate cardiovascular responses and blood pressure in obese individuals [17].
Angiotensin II has been established to affect cardiovascular function such as homeostasis and hypertension. Angiotensin II receptors AT1 are expressed by human platelets [19]. Studies have demonstrated that angiotensin II can induce a change in the shape of human platelets [20].
Acylation stimulating protein
The production of acylation stimulating protein (ASP) occurs through a two-step process. The process involves three proteins of the alternate complement system: factor B, C3 and adipsin. All three proteins are synthesized and secreted by adipocytes [21].
It has a major effect on the increase of lipogenesis by the activity of diacylglycerol acyltransferase (DGAT) and translocation of glucose transporter type 4 (GLUT4) in glycerol 3-phosphate [21, 22]. Acyl stimulating protein in plasma increases with meals and facilitates triglyceride synthesis and storage. ASP deficiency increases levels of postprandial fatty acid and decreases triglyceride synthesis and weight gain [23].
In cases of obesity, type 2 diabetes (T2D), and cardiovascular disease in humans, levels of ASP increase, but they decrease with exercise or weight loss. After eating, the subcutaneous adipose tissue produces more ASP, which has been linked to the local trapping of fatty acids. Additionally, it has been suggested that an ASP-resistant state, similar to insulin resistance, contributes to the disturbed adipose tissue metabolism and dyslipidemia seen in diabetes and cardiovascular disease. Interestingly, ASP levels tend to decrease with age, a phenomenon observed in humans, with children having higher ASP values than adults [22, 24].
Visfatin
Visfatin is a protein that weighs 52 kDa and is found mainly in visceral adipose tissue. It is also known as pre-B cell colony-enhancing factor (PBEF). Adipocytes are the main producers of visfatin, but macrophages in visceral adipose tissue also produce it, albeit in small amounts, and it is also present in subcutaneous adipose tissue. The expression of visfatin mRNA significantly increases during the process of preadipocyte differentiation into adipocytes [25, 26].
Studies have demonstrated that visfatin primarily functions in energy metabolism and innate immunity, and it is now considered a pro-inflammatory adipocytokine. Its properties activate leukocytes and promote the production of TNF-α and IL-6 [26, 27].
Within cells, visfatin serves as a nicotinamide phosphoribosyltransferase involved in the salvage pathway of NAD+ biosynthesis. As such, it can regulate the cellular levels of NAD+, thereby influencing cell energy metabolism and the activity of NAD+/NADH dependent enzymes [23].
Recent research has shown that visfatin has insulin mimetic effects on cultured adipocytes, myocytes, and hepatocytes. It can also lower plasma glucose levels in mice by binding to and activating the insulin receptor [28].
Plasminogen activator inhibitor 1 (PAI-1)
Plasminogen activator inhibitor 1 (PAI-1) is another factor involved in microvascular events. The gene responsible for PAI-1 is located on chromosome 7q21.3-q22. PAI-1 is a glycoprotein weighing 45-kDa, and it contains between 379 to 381 amino acids. Although endothelial and vascular smooth muscle cells are believed to be the primary sources of PAI-1, other cells, including platelets, hepatocytes, mesangial cells, fibroblasts, monocytes, macrophages, adipocytes, and stromal cells within adipose tissue, have been found to secrete the serpin [29].
The contribution of adipose production to circulating PAI-1 increases as the adipose tissue mass and fat cell size increase. Research has shown that visceral adipose tissue has a greater capacity to produce PAI-1 than subcutaneous adipose tissue. Studies on human adipocytes indicate that PAI-1 synthesis is upregulated by insulin, glucocorticoids, angiotensin II, certain fatty acids, and most significantly, by cytokines such as TNF-α and transforming growth factor-β. On the other hand, catecholamines decrease PAI-1 production [29, 30].
PAI-1 is a protein involved in fibrinolysis and is affected by obesity [31]. As visceral adiposity increases, plasma PAI-1 levels also increase, suggesting that PAI-1 may serve as the link between central obesity and cardiovascular disease. This protein can shift the balance between fibrinolysis and fibrinogenesis, leading to vascular remodeling and the development of atherosclerosis [23]. Dysfunctions in the endocrine system and impaired auto-/paracrine functions at the adipocyte level may contribute to this disruption of the fibrinolytic system, ultimately increasing the risk of cardiovascular disease [30].
Adipose tissue serves not only as a storage site for excess energy but also as an endocrine organ, producing a range of signals including cytokines, hormones, and growth factors. These signals impact neighboring cells as well as target tissues involved in energy metabolism and other physiological and pathological processes. Much research in this area has focused on two prototypic adipokines, leptin and adiponectin, which have beneficial effects on insulin action and lipid metabolism. Obesity is characterized by an increase in fat cell number and size, or a combination of the two. Recent evidence suggests that low-grade inflammation within adipose tissue contributes to the dysregulation of adipocytokine production, leading to the pathophysiology of metabolic syndrome (MetS). In obesity, inflamed macrophages infiltrate adipose tissue, releasing TNF-α and IL-6, linking obesity, inflammation, and insulin resistance. Understanding the signaling pathways by which adipokines control metabolism is essential to developing new therapies for adipose tissue-related diseases.
References
- Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2(4):282-286. doi:10.1093/embo-reports/kve071
- Kiess W, Petzold S, Töpfer M, et al. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab. 2008;22(1):135-153. doi:10.1016/j.beem.2007.10.002
- Laclaustra M, Corella D, Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis. 2007;17(2):125-139. doi:10.1016/j.numecd.2006.10.005
- Bernlohr DA, Jenkins AE, Bennaars AA. Adipose tissue and lipid metabolism. In: Vence JE, Vence D, editors. Biochemistry of lipids, lipoproteins and membranes. 4th ed. Elsevier Science: Amsterdam; 2002. pp. 263–89
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91
- Zhang Y et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432
- Li MD. Leptin and beyond: An odyssey to the central control of body weight. The Yale Journal of Biology and Medicine. 2011;84(1):1-7
- La Cava A, Matarese G. The weight of leptin in immunity. Nature Reviews. Immunology. 2004;4(5):371-379
- Bluher S, Mantzoros CS. Leptin in reproduction. Current Opinion in Endocrinology, Diabetes, and Obesity. 2007;14(6):458-464
- Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nature Reviews. Immunology. 2006;6(10):772-783
- Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding [published correction appears in J Clin Invest. 2006 May;116(5):1457]. J Clin Invest. 2006;116(1):115-124. doi:10.1172/JCI24335
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129-139. doi:10.1016/j.mce.2009.08.018
- Kaser S, Tatarczyk T, Stadlmayr A, et al. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur J Clin Invest. 2008;38(11):827-834. doi:10.1111/j.1365-2362.2008.02028.x
- Palanivel R, Fang X, Park M, et al. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75(1):148-157. doi:10.1016/j.cardiores.2007.04.011
- Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149(5):2270-2282. doi:10.1210/en.2007-1561
- Diamond F. The endocrine function of adipose tissue. Growth Genetics Horm. 2002;18:17–23
- Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55(10):2688-2697. doi:10.2337/db05-1404
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11(8):327-332. doi:10.1016/s1043-2760(00)00301-5
- Touyz RM, Schiffrin EL. Effects of angiotensin II and endothelin-1 on platelet aggregation and cytosolic pH and free Ca2+ concentrations in essential hypertension. Hypertension. 1993;22(6):853-862. doi:10.1161/01.hyp.22.6.853
- Jagroop IA, Mikhailidis DP. Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. J Hum Hypertens. 2000;14(9):581-585. doi:10.1038/sj.jhh.1001102
- Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609(2):127-143. doi:10.1016/s0005-2736(02)00686-7
- Paglialunga S, Fisette A, Yan Y, et al. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008;294(3):E521-E529. doi:10.1152/ajpendo.00590.2007
- Manolescu B, Stoian I, Atanasiu V, Busu C, Lupescu O. Review article: The role of adipose tissue in uraemia-related insulin resistance. Nephrology (Carlton). 2008;13(7):622-628. doi:10.1111/j.1440-1797.2008.01022.x
- Cianflone K, Lu H, Smith J, Yu W, Wang H. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin Endocrinol (Oxf). 2005;62(5):567-572. doi:10.1111/j.1365-2265.2005.02260.x
- Olszanecka-Glinianowicz M, Kocełak P, Nylec M, Chudek J, Zahorska-Markiewicz B. Circulating visfatin level and visfatin/insulin ratio in obese women with metabolic syndrome. Arch Med Sci. 2012;8(2):214-218. doi:10.5114/aoms.2012.28547
- Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92(2):666-672. doi:10.1210/jc.2006-1303
- Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748-1758. doi:10.4049/jimmunol.178.3.1748
- Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81(2):370-380. doi:10.1093/cvr/cvn288
- Correia ML, Haynes WG. A role for plasminogen activator inhibitor-1 in obesity: from pie to PAI?. Arterioscler Thromb Vasc Biol. 2006;26(10):2183-2185. doi:10.1161/01.ATV.0000244018.24120.70
- Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28(11):1357-1364. doi:10.1038/sj.ijo.0802778
- Mertens I, Van Gaal LF. Visceral fat as a determinant of fibrinolysis and hemostasis. Semin Vasc Med. 2005;5(1):48-55. doi:10.1055/s-2005-871741
Kindly Let Us Know If This Was helpful? Thank You!


