There are eleven known pathophysiologic defects that Type 2 diabetes mellitus (T2DM) is characterized by. These eleven features are often referred to as the “egregious eleven”.
1. Pancreatic beta cell dysfunction
The beta cells in the pancreas are responsible for producing and secreting insulin in response to sugar levels in the blood. Beta cell dysfunction is a key feature of type 2 diabetes mellitus, and it leads to hyperglycemia by impairing the body’s ability to produce and/or release insulin. Insulin resistance is a major contributing factor to beta cell dysfunction, and it occurs when the body’s cells become resistant to the effects of insulin.
This resistance can be caused by a number of factors, including obesity, genetics, and certain medications. When the body’s cells are resistant to insulin, they cannot effectively uptake glucose from the bloodstream, which leads to elevated blood sugar levels. Over time, this can damage the beta cells and cause them to dysfunction (progressive beta cell death). In addition, when the beta cells are not functioning properly, they cannot secrete enough insulin to keep blood sugar levels under control, which further exacerbates the problem.
2. Loss of the incretin effect
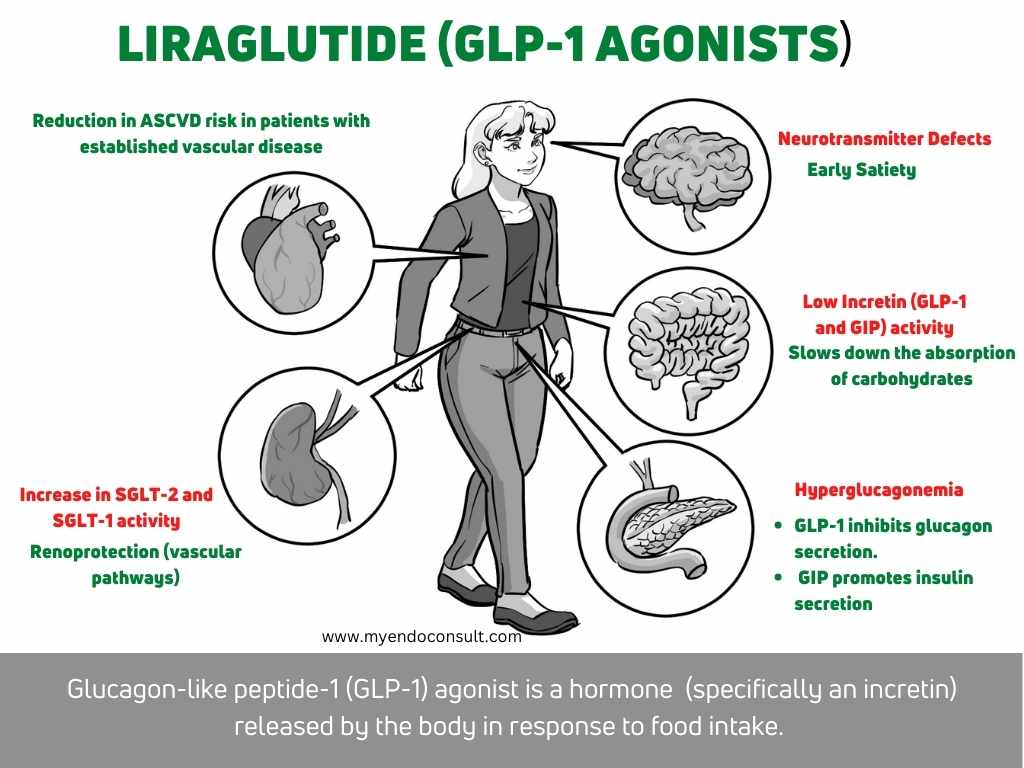
The two main types of incretins are glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). GIP is produced by K and L cells of the intestine. GIP and GLP-1 work together to regulate blood sugar levels after a meal. GIP promotes insulin secretion, while GLP inhibits glucagon release.
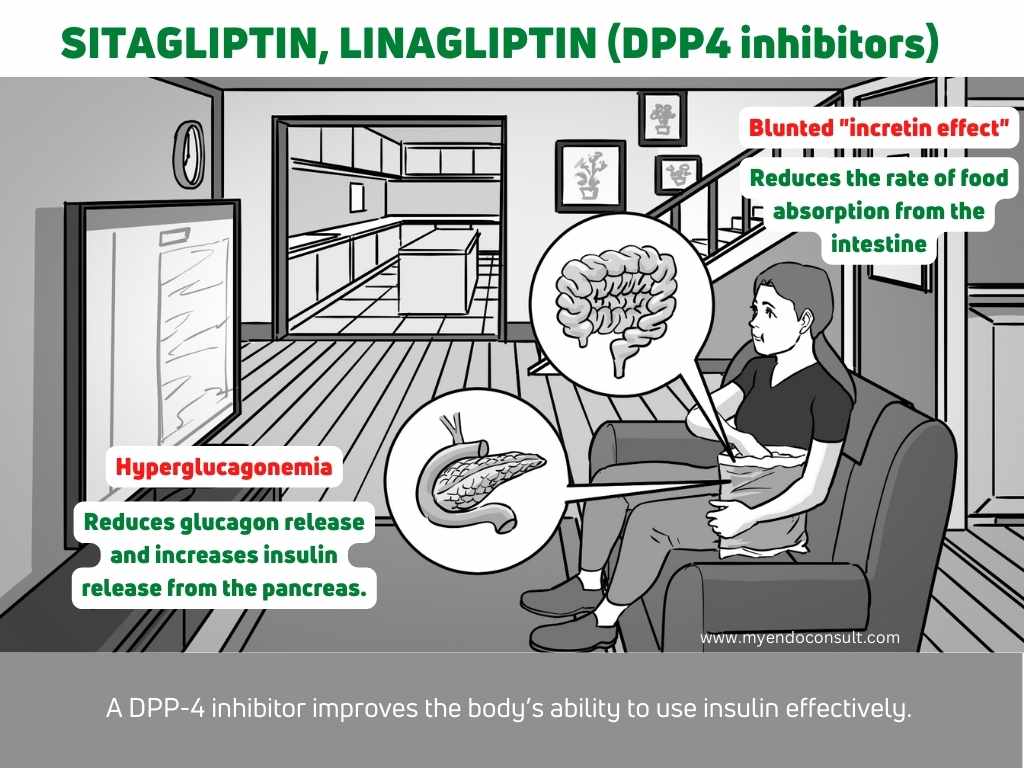
The incretin effect is a major contributor to normal glucose metabolism. It is the enhancement of glucose-dependent insulin secretion and suppression of glucagon secretion in response to an oral glucose load. The incretin effect is mediated by the gut hormone GLP-1. GLP-1 is secreted by L cells in the intestine in response to food intake. It acts on pancreatic beta cells to stimulate insulin secretion and on liver cells to suppress glucagon secretion. GLP-1 also delays gastric emptying and reduces food intake (early satiety). In type 2 diabetes, there is a loss of the incretin effect, which leads to uncontrolled hyperglycemia. This loss of the incretin effect is thought to be due to a combination of factors, including a decrease in GLP-1 secretion and an increase in GLP-1 degradation. The loss of the incretin effect contributes to the progressive deterioration of glycemic control in type 2 diabetes and is a major therapeutic target for the treatment of this disease.
3. Pancreatic alpha cell dysfunction
Pancreatic alpha cell dysfunction is a condition in which the cells that produce glucagon (a hormone that regulates blood sugar levels) do not function properly. This often leads to elevated levels of glucose in the blood, which can lead to type 2 diabetes. Hyperglucagonemia (high levels of glucagon in the blood) is a common complication of pancreatic alpha cell dysfunction and is thought to contribute to the development of type 2 diabetes.
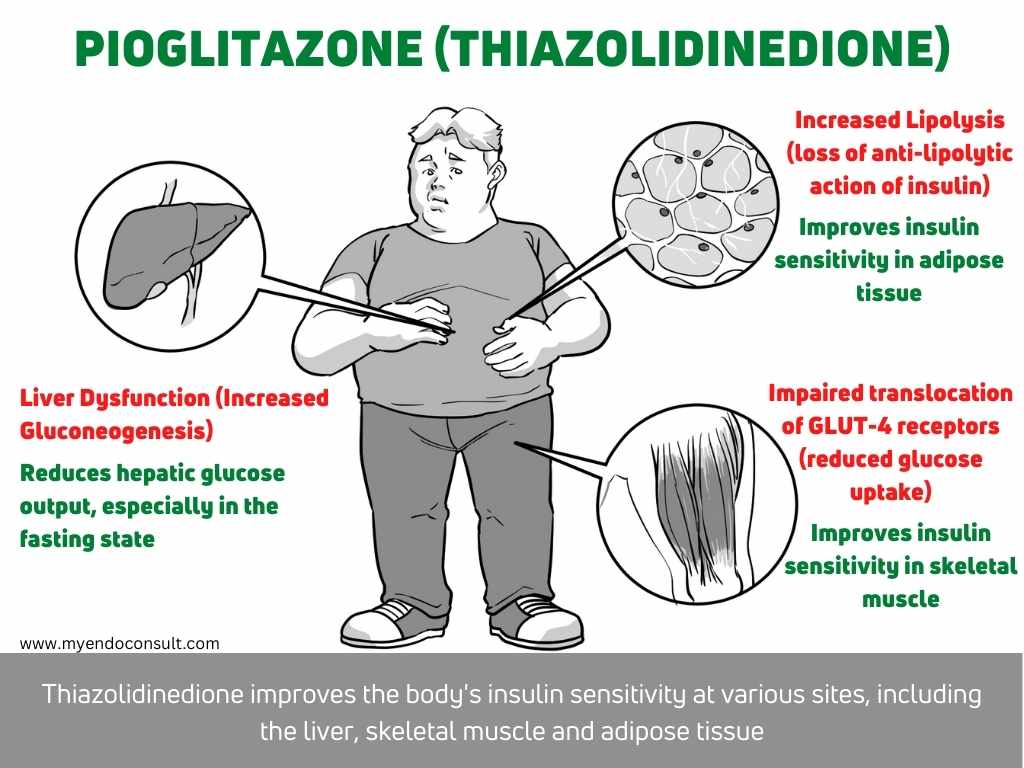
4. Adipose tissue dysfunction (insulin resistance)
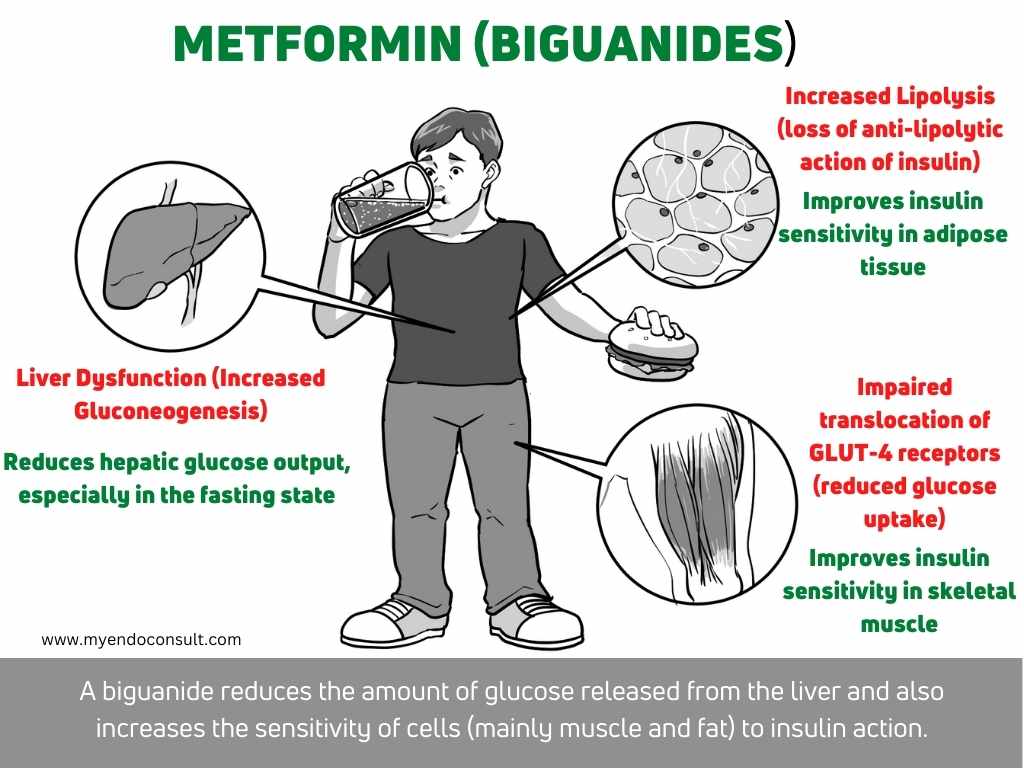
Adipose tissue dysfunction is a major contributing factor to the development of type 2 diabetes. In lipolysis, triglycerides are broken down into free fatty acids and glycerol, and free fatty acids are subsequently released into the blood. Furthermore, free fatty acids are then taken up by the liver, where they are used for energy or stored as fat. With adipose tissue dysfunction, lipolysis is impaired and free fatty acids are not properly metabolized. This results in increased levels of free fatty acids in the blood, which leads to insulin resistance and type 2 diabetes.
5. Muscle tissue dysfunction (insulin resistance)
Muscle tissue dysfunction is a major contributor to the development of insulin resistance and type 2 diabetes mellitus (T2DM). In skeletal muscle, lipolysis is increased in the setting of insulin resistance and T2DM, which results in elevated concentrations of free fatty acids (FFA). The accumulation of FFA in muscle cells leads to impaired glucose uptake through the insulin-sensitive GLUT-4 transporter, contributing to peripheral insulin resistance. In addition, FFA accumulation promotes lipotoxicity and oxidative stress, further exacerbating muscle tissue dysfunction and insulin resistance. Thus, targeting muscle tissue dysfunction represents a promising therapeutic strategy for the treatment of T2DM.
6. Liver dysfunction (insulin resistance)
Hepatic dysfunction is a pathophysiologic defect that can lead to type 2 diabetes as well. Under normal circumstances, the liver regulates glucose levels by gluconeogenesis (the process of synthesizing glucose from non-carbohydrate sources) and glycolysis (the breakdown of glucose to generate energy). However, in hepatic dysfunction, the liver is unable to properly regulate these processes, leading to increased hepatic glucose output and decreased insulin sensitivity. This can eventually lead to type 2 diabetes if left untreated. The most common cause of hepatic dysfunction is fatty liver disease, which is often linked to obesity and insulin resistance.
7. Brain dysfunction
The brain normally regulates appetite by sending signals to the body that tell it when to feel hungry and when to feel full. However, in people with type 2 diabetes, this process is disrupted, leading to higher levels of hunger and cravings for foods that are high in sugar and fat. This can lead to weight gain and eventually to type 2 diabetes. Although the exact cause of this appetite dysregulation is not yet known, it is thought to be due to a combination of genetic and environmental factors.
8. Colon dysfunction (Gut dysbiosis)
The gut microbiome is a complex ecosystem of microbes that resides in the human gastrointestinal tract. This microbiota plays a critical role in gut health and has been shown to be involved in the pathophysiology of various diseases, including type 2 diabetes. Studies have shown that gut dysbiosis, or an imbalance in gut microbial composition, is associated with type 2 diabetes. This gut dysbiosis can lead to impaired gut barrier function and increased inflammation, both of which are thought to contribute to the development of type 2 diabetes. Additionally, gut dysbiosis has been shown to alter glucose metabolism, by blunting the ability of the intestines to produce incretins. This leads to significant impairment of the previously described “incretin effect”.
9. Immune system dysfunction
It is now clear that immune dysregulation and inflammation play a significant role in the pathogenesis of type 2 diabetes. For example, chronic low-grade inflammation has been shown to contribute to the development of insulin resistance, while pro-inflammatory cytokines have been linked to beta-cell dysfunction and death. These findings suggest that targeting inflammation may be a promising therapeutic strategy for treating T2DM.
10. Low amylin levels
Amylin is a peptide hormone that is secreted by the pancreas along with insulin. Amylin plays several important roles in regulating glucose metabolism, such as slowing gastric emptying and promoting satiety. In individuals with type 2 diabetes mellitus, amylin levels are often low, which can lead to hyperglycemia
11. Kidney dysfunction
In type 2 diabetes, renal glucose handling is impaired, and there is an increase in SGLT-2 activity and SGLT-1-mediated glucose reabsorption.
Choosing pharmacotherapy based on pathophysiologic defects
| Pathophysiologic defect | Medication |
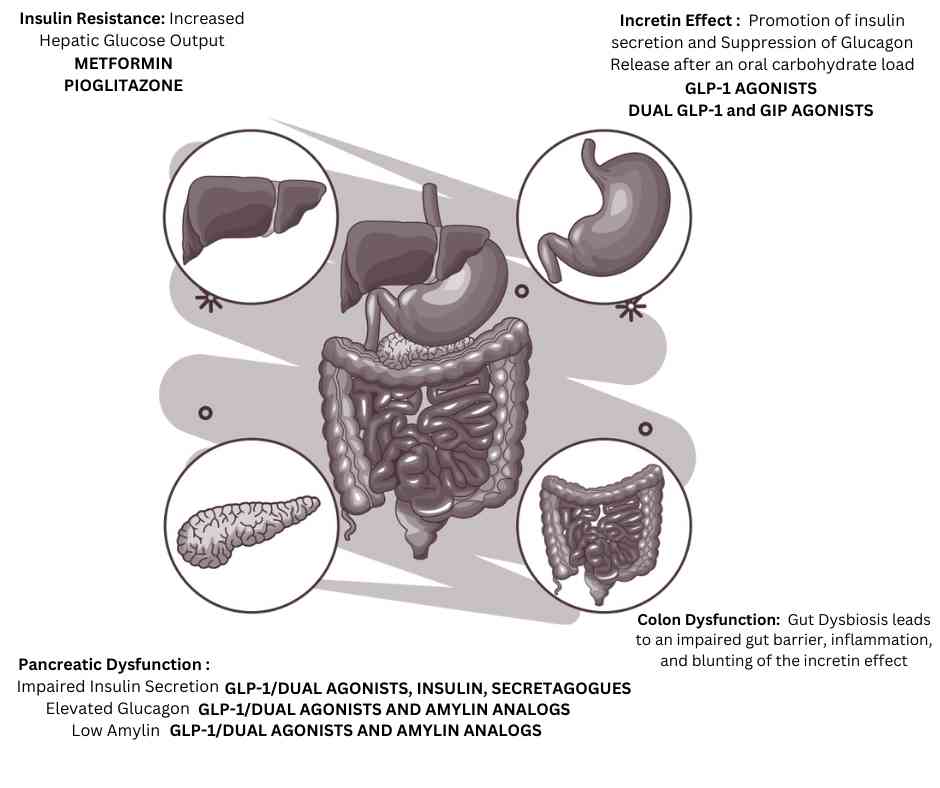
| Pancreatic beta cell dysfunction | GLP-1 analogs, Dual GLP-1 and GIP analogs, Ranolazine |
| Loss of the incretin effect | GLP-1 analogs, Dual GLP-1 and GIP analogs |
| Pancreatic alpha cell dysfunction | GLP-1 analogs, Dual GLP-1 and GIP analogs Pramlintide (amylin analog) |
| Adipose tissue dysfunction (insulin resistance) | Metformin Thiazolidinediones |
| Muscle tissue dysfunction (insulin resistance) | Metformin Thiazolidinediones |
| Liver dysfunction (insulin resistance) | Metformin Thiazolidinediones |
| Brain dysfunction | GLP-1 analogs, Dual GLP-1 and GIP analogs Dopamine agonists |
| Colon dysfunction (Gut dysbiosis) | GLP-1 analogs, Dual GLP-1 and GIP analogs Metformin Probiotics |
| Immune system dysfunction | GLP-1 analogs, Dual GLP-1 and GIP analogs |
| Low amylin levels | GLP-1 analogs, Dual GLP-1 and GIP analogs Pramlintide (amylin analog) |
| Kidney dysfunction | SGLT-2 inhibitors |
What are the egregious eleven of diabetes mellitus?
The egregious eleven refers to the 11 core pathophysiologic defects of type 2 diabetes mellitus.
- Pancreatic beta cell dysfunction
- Loss of the incretin effect
- Pancreatic alpha cell dysfunction
- Adipose tissue dysfunction (insulin resistance)
- Muscle tissue dysfunction (insulin resistance)
- Liver dysfunction (insulin resistance)
- Brain dysfunction
- Colon dysfunction (Gut dysbiosis)
- Immune system dysfunction
- Low amylin levels
- Kidney dysfunction
References
Schwartz SS, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med. 2016 Nov;128(8):828-838.
Download all of the above infographics for your personal use only. If you require high-quality prints, visit this page. Thanks.
Kindly Let Us Know If This Was helpful? Thank You!


