Insulin Resistance
Insulin resistance is a complex pathophysiological condition characterized by impaired biological response of body tissues, such as adipose tissue, liver, and muscle, to insulin stimulation. As a consequence of this, glucose uptake and disposal at the target tissues are hampered, leading to compensatory hyperinsulinemia due to increased pancreatic synthesis of insulin by beta cells. Insulin resistance is a key contributor to the development of metabolic disorders, such as non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), and metabolic syndrome
Epidemiological data reveal a consistent association between insulin resistance and metabolic syndrome, and while the condition is prevalent across all races, inter-racial comparisons are limited. A national survey conducted by the National Cholesterol Education Program Adult Treatment Panel III found that approximately 24% of adults over the age of 20 in the United States exhibit symptoms of insulin resistance syndrome.
Insulin signaling in normal physiology
Insulin signaling is a complex process by which insulin, a hormone produced by the pancreas, regulates glucose homeostasis and other metabolic processes in the body. Insulin signaling plays a critical role in the development of insulin resistance, type 2 diabetes, and other metabolic disorders, making it an important area of research and study.
Insulin is secreted by beta cells of the pancreas in response to elevated levels of glucose in the blood. Once insulin is released into the bloodstream, it binds to specific receptors on target cells, which activates a cascade of signaling pathways that lead to the uptake and utilization of glucose in the body.
The primary signaling pathway of insulin begins with the binding of insulin to its receptor, which activates the receptor’s tyrosine kinase activity. This, in turn, activates a series of intracellular signaling molecules, including insulin receptor substrate (IRS) proteins, phosphatidylinositol-3 kinase (PI3K), and Akt (also known as protein kinase B).
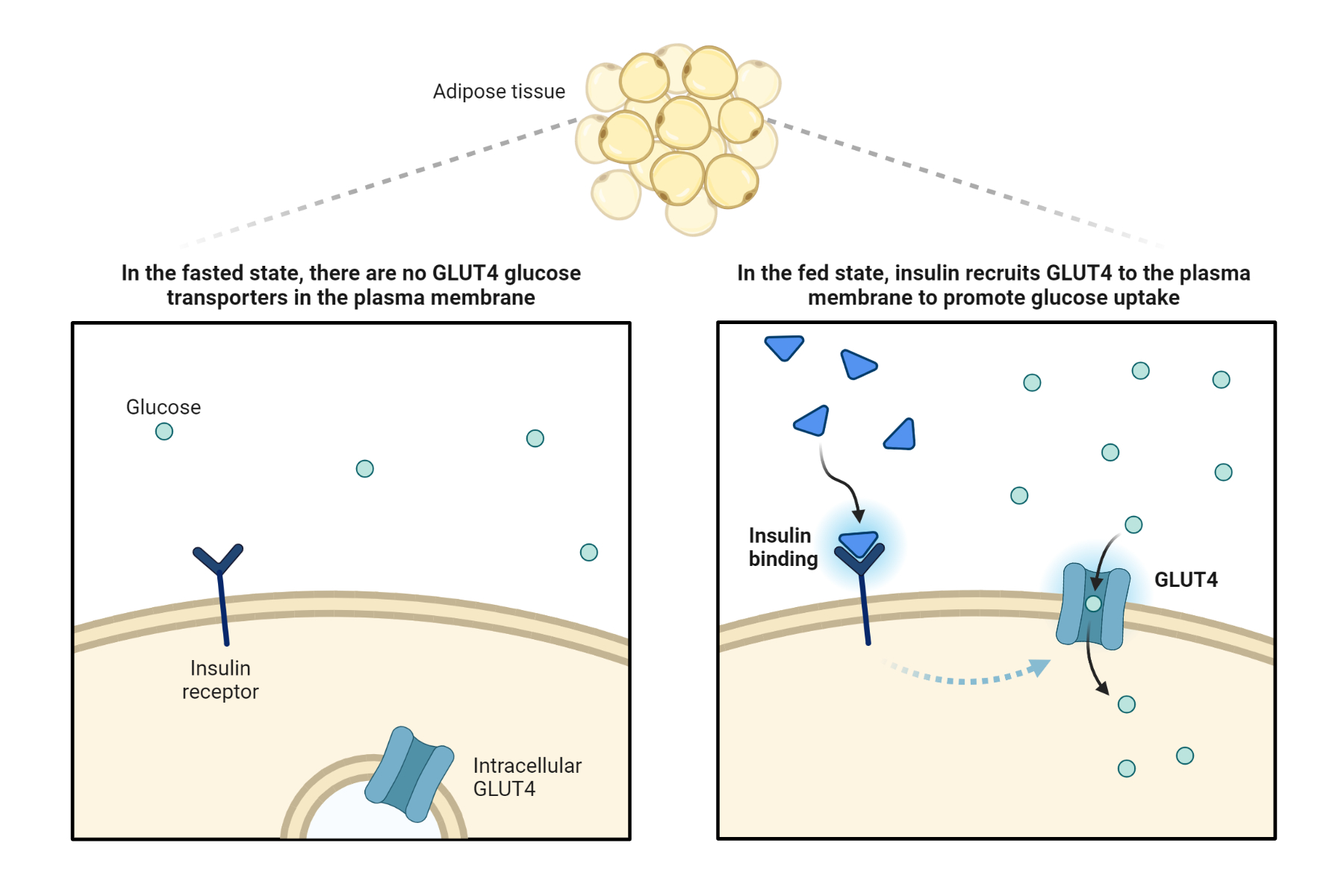
Activation of Akt results in the translocation of glucose transporter 4 (GLUT4) from intracellular vesicles to the plasma membrane, allowing glucose to enter the cell. Insulin signaling also leads to the activation of other signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, which plays a role in cell growth and proliferation.
Insulin resistance, a condition characterized by impaired insulin signaling, leads to elevated blood glucose levels and is a hallmark of type 2 diabetes. Insulin resistance can result from a variety of factors, including genetic predisposition, lifestyle factors such as poor diet and physical inactivity, and chronic inflammation.
In addition to its role in glucose metabolism, insulin signaling plays a critical role in lipid metabolism, protein synthesis, and cellular differentiation. Disruptions in insulin signaling have been linked to a variety of metabolic disorders, including obesity, non-alcoholic fatty liver disease (NAFLD), and cardiovascular disease.
Recent research has focused on developing new therapies to improve insulin signaling and treat metabolic disorders. These include the use of insulin sensitizers, which improve insulin sensitivity in target tissues, as well as the use of novel compounds that target specific components of the insulin signaling pathway.
Pathogenesis of insulin resistance in type 2 diabetes mellitus
Insulin resistance is a condition in which the body’s cells become less responsive to insulin, leading to elevated blood sugar levels. The pathogenesis of insulin resistance involves a complex interplay of multiple signaling pathways in various tissues, including the liver, muscle, adipose tissue, and pancreas.
In liver cells, insulin resistance is associated with impaired insulin signaling through the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. This leads to reduced glucose uptake and increased glucose production, contributing to hyperglycemia. Insulin resistance in the liver is also linked to increased production of glucose via the cAMP/PKA signaling pathway.
In muscle cells, insulin resistance is characterized by defects in glucose transport and utilization. This is due in part to impaired insulin signaling through the PI3K/Akt pathway, which normally stimulates glucose uptake and metabolism. In addition, insulin resistance in muscle may be related to inflammation, oxidative stress, and mitochondrial dysfunction, which can impair energy metabolism and contribute to insulin resistance.
Adipose tissue plays a critical role in insulin signaling through its production of adipokines, including adiponectin and leptin. Insulin resistance in adipose tissue is associated with impaired secretion of adipokines, which can interfere with insulin signaling in other tissues. In addition, excessive accumulation of lipids in adipose tissue can lead to inflammation and oxidative stress, which can contribute to insulin resistance.
The pancreas is also involved in insulin resistance, particularly in cases of type 2 diabetes. Insulin resistance in the pancreas may be related to defects in insulin secretion, which can result from impaired signaling through the PI3K/Akt pathway. In addition, chronic exposure to high levels of glucose and lipids can lead to beta-cell dysfunction and apoptosis, contributing to the development of diabetes.
Overall, insulin resistance is a complex condition that involves multiple signaling pathways and mechanisms. Advances in understanding the pathogenesis of insulin resistance are critical for developing new treatments and preventive strategies for diabetes and related conditions. Ongoing research is focused on identifying new targets for therapy and developing more effective approaches to managing insulin resistance and related metabolic disorders.
Causes and Risk Factors of Insulin Resistance
Several risk factors have been identified for the development of insulin resistance, including age, physical inactivity, body fat mass, abdominal adiposity, and gender. However, studies have reported inconsistent associations between dairy product ingestion and the development of insulin resistance. Further, prospective studies have suggested that high vascular adipose tissue and inflammatory markers, such as tumor necrosis factor-alpha (TNF-α), high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6), are additional risk factors that can increase the likelihood of developing insulin resistance.
Table 1. Etiology of Insulin Resistance
| Acquired Causes | Hereditary Causes |
| · Aging · Physical inactivity · Glucose toxicity · Increased sodium in the diet · Excess dysfunctional adipose tissue · Nutritional imbalance · Lipotoxicity due to excess free fatty acids in the circulation · Medications (exogenous insulin, glucocorticoids, protease inhibitors, anti-adrenergic, and atypical antipsychotics) | · PCOS (Polycystic ovarian syndrome)) · Myotonic dystrophy · Werner syndrome · Ataxia telangiectasia · Lipodystrophy · Rabson-Mendenhall syndrome · Lipodystrophy · Alstom syndrome |
Types of Insulin Resistance
Insulin resistance is a complex pathological state that can be classified into different types based on its clinical features and the site of insulin dysfunction. Two characteristic types of insulin resistance have been identified, known as Type-A and Type-B insulin resistance.
Type-A insulin resistance is characterized by severe insulin resistance without the presence of anti-insulin antibodies. Clinical features of Type-A insulin resistance include acanthosis nigricans, abnormal glucose homeostasis, and ovarian virilization, and typically occur prior to middle age.
Type-B insulin resistance is characterized by acanthosis nigricans, abnormal glucose homeostasis, and ovarian hyperandrogenism, which can lead to the formation of anti-insulin antibodies, usually during middle age.
Additionally, insulin resistance can be classified based on the site of dysfunction of insulin. This includes pre-receptor, receptor, and post-receptor types. Pre-receptor insulin resistance refers to insulin resistance due to abnormalities in insulin synthesis or secretion. Receptor insulin resistance occurs when insulin receptors on cell surfaces are impaired, leading to decreased insulin sensitivity. Post-receptor insulin resistance refers to defects in insulin signaling pathways after insulin has bound to its receptors.
Clinical features of Insulin Resistance
Insulin resistance is a complex pathological state that can present in several ways. The following are the varying clinical presentations of insulin resistance:
· Polycystic ovary syndrome (PCOS)
· Features of PCOS, including menstrual irregularities, acne, alopecia, and hirsutism
· Elevated blood pressure
· The metabolic syndrome
· Characteristic features of genetic syndromes, including insulin resistance syndrome
· Prediabetes (either impaired fasting or postprandial glycemia)
· Overt Type 2 diabetes mellitus (T2DM)
· Asymptomatic patients with hyperlipidemia, obesity, or hypertension
· Acanthosis nigricans
· Xanthelasma or xanthoma
· Type-A insulin resistance
· Type-B insulin resistance
· Increased waist circumference
Clinical Evaluation of Insulin Resistance
Insulin sensitivity is an important measure of the body’s response to insulin and can provide valuable insights into the development and progression of insulin resistance. Several indices are commonly used to assess insulin sensitivity or resistance in clinical practice, including the Matsuda index, the quantitative insulin sensitivity check index (QUICKI), and the homeostasis model assessment-insulin resistance (HOMA-IR).
HOMA-IR is a simple, minimally invasive index that uses fasting steady-state insulin and glucose levels to predict precise insulin sensitivity.
A normal HOMA-IR level is < 2.5, and the formula for calculating HOMA-IR is (Fasting Insulin×Fasting Glucose)/22.5.
One of the limitations of HOMA-IR is that it requires further validation in individuals who are administered insulin.
QUICKI is a consistent, minimally invasive index that uses log-transformed fasting insulin and glucose levels to predict insulin sensitivity.
Normal QUICKI levels include 0.304 in diabetic individuals, 0.382 in non-obese individuals, and 0.331 in obese individuals.
The formula for calculating QUICKI is 1/(log Insulin + log Glucose).
One of the disadvantages of QUICKI is that interlaboratory differences in insulin assay can affect its accuracy.
Complications of Insulin Resistance
Insulin resistance is a complex condition that can have serious consequences for the health of affected individuals. The vascular complications of insulin resistance can be categorized into microvascular and macrovascular complications, including retinopathy, neuropathy, nephropathy, cerebrovascular accidents, peripheral artery disease, and coronary artery disease. Mortality outcomes in insulin resistance are independent of body mass, hypertension, and dyslipidemia, and are often linked to an increased risk of cardiovascular complications and chronic kidney disease.
Prevention and treatment of insulin resistance involve three levels of intervention: primary, secondary, and tertiary. Primary prevention involves public education about the importance of regular health monitoring and healthy lifestyle modifications to prevent the development of metabolic syndrome and diabetes. Secondary prevention includes laboratory screening for diabetes and insulin resistance to administer early intervention. Tertiary prevention aims to decrease mortality and morbidity of insulin resistance complications through bariatric surgery and intensive medical intervention.
Exercise, particularly aerobic training, has been shown to reduce insulin resistance and prevent the development of type 2 diabetes and metabolic syndrome. Other effective measures for high-risk adults include dietary therapy, personalized programs, and education, support, and medication. Metformin is the first-line medication for type 2 diabetes and PCOS treatment, while glucagon-like peptide 1 (GLP-1) receptor agonists, DPP-4 inhibitors, and thiazolidinediones (TZDs) are also used to manage insulin resistance.
Surgical interventions, including bypass, gastric sleeve, and banding, may be required for obese individuals who qualify for surgery. Bariatric surgery reduces excess fat and improves insulin sensitivity, leading to better outcomes for patients.
In conclusion, insulin resistance is a common and preventable condition that requires close monitoring and intervention to prevent or manage its complications. Future research studies should adopt a clinical trial and/or longitudinal cohort design to assess the outcomes of different interventional modalities, considering the comparative effectiveness of these interventions in different populations based on gender, race, age, and co-morbidities.
References
- Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front Endocrinol (Lausanne). 2019;10:80.
- Brown JC, Harhay MO, Harhay MN. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis. Nutr Metab Insights. 2019;12:1178638819831712.
- Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res. 2019;42(5):383-92.
- Fahed M, Abou Jaoudeh MG, Merhi S, Mosleh JMB, Ghadieh R, Al Hayek S, et al. Evaluation of risk factors for insulin resistance: a cross-sectional study among employees at a private university in Lebanon. BMC endocrine disorders. 2020;20:1-14.
- Sundström-Poromaa I, Thu WPP, Kramer MS, Logan S, Cauley JA, Yong EL. Risk factors for insulin resistance in midlife Singaporean women. Maturitas. 2020;137:50-6.
- Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian journal of endocrinology and metabolism. 2015;19(1):160.
- Adamczak M, Ritz E, Wiecek A. Chapter 3 – Carbohydrate metabolism in chronic renal disease. In: Kopple JD, Massry SG, Kalantar-Zadeh K, Fouque D, editors. Nutritional Management of Renal Disease (Fourth Edition): Academic Press; 2022. p. 29-41.
- Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev Med. 2016;93:211-8.
- Aroda VR, Knowler WC, Crandall JP, Perreault L, Edelstein SL, Jeffries SL, et al. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60(9):1601-11.
Kindly Let Us Know If This Was helpful? Thank You!


