A closer look at the female reproductive system
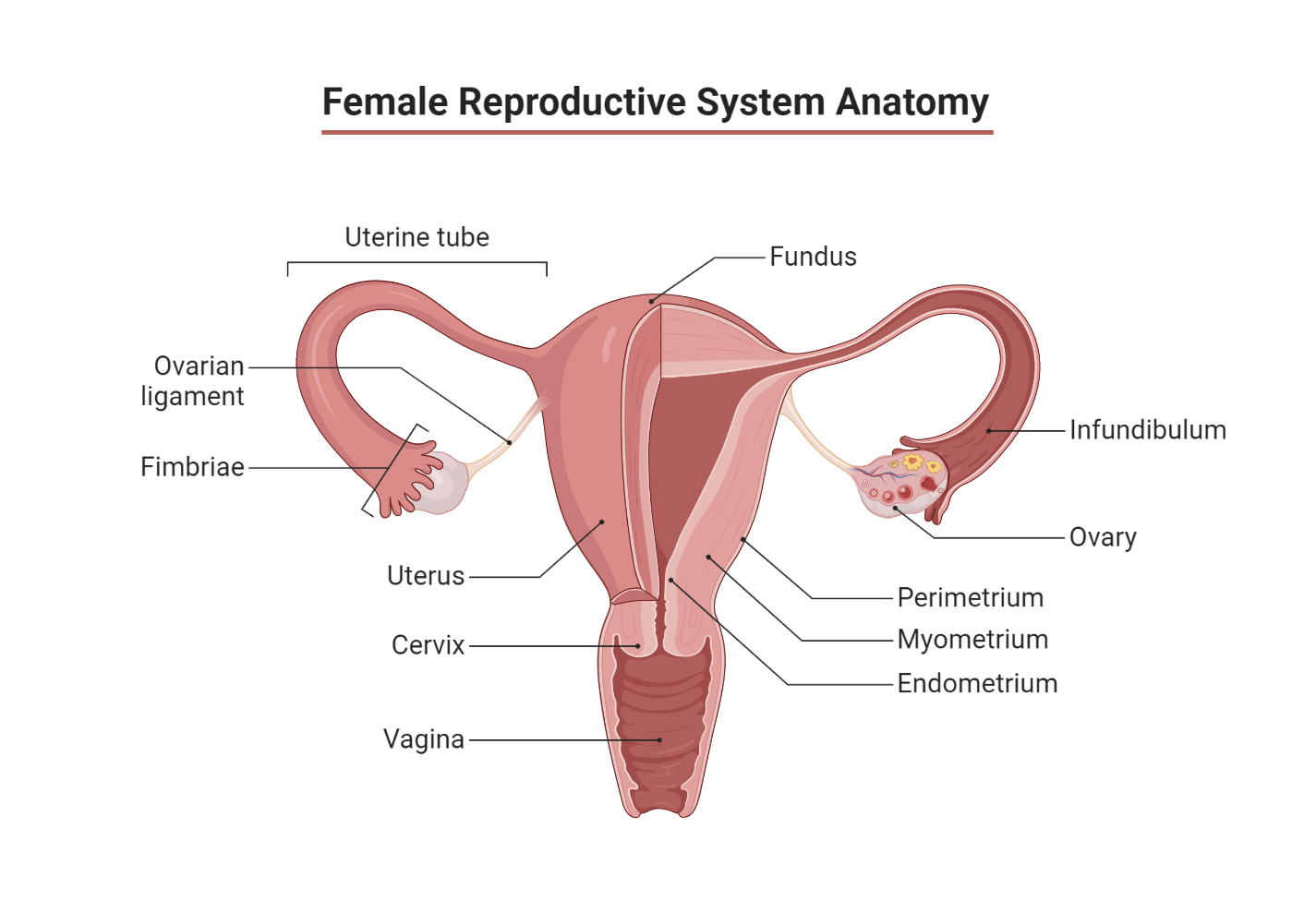
Nestled on either side of the uterus, close to the open ends of the oviducts or fallopian tubes, the two ovaries sit in the pelvis. Each weighing around 15 grams, the ovaries are wrapped in a protective connective tissue capsule. The cortex of the ovaries harbors thousands of primordial follicles—around 200,000 at puberty. These follicles are embedded in a supportive tissue known as the stroma.
Every primordial follicle consists of an oocyte or ovum encapsulated by a single layer of granulosa cells. During the female’s reproductive lifespan, approximately 450 of these follicles mature and expel an oocyte, with the remainder undergoing a process of spontaneous degeneration called atresia. By menopause, the time when menstruation ceases, the ovaries typically contain only a handful of primordial follicles.
Understanding the ovarian cycle and hormone release
Throughout each menstrual cycle, ovarian follicles progress through three developmental stages, culminating in the formation of a mature Graafian follicle. The onset of each menstrual cycle—the follicular phase—sees 10-20 primordial follicles expand to form secondary follicles, a process called recruitment, under the influence of rising follicle-stimulating hormone (FSH) concentrations.
However, by around day 6, generally, only one follicle becomes dominant and achieves full maturation, while the others regress and become atretic. At midcycle, or day 14, the mature Graafian follicle migrates to the ovary’s edge and bursts, releasing the oocyte into the abdominal cavity. This event, known as ovulation, allows the oocyte to enter one of the fallopian tubes.
If the ovum is fertilized during its 3-4 day journey down the fallopian tube, cell division commences—initially at the blastocyst stage—before implantation in the uterus, followed by further development at the trophoblast stage. Trophoblast cells, in conjunction with adjacent blastocyst and endometrial cells, divide rapidly to form the placenta. If the ovum is not fertilized within approximately 72 hours, it dies and is eventually expelled through the vagina.
Summary of the follicular maturation process
- Primary (or primordial) follicle: This is an immature oocyte, approximately 25mm in diameter, surrounded by a single layer of follicular epithelial cells. After the primary follicle stage, we move on to the next stage in the journey of follicular maturation.
- Secondary follicle: At this stage, the oocyte, now roughly 500mm in diameter, enlarges under the influence of FSH. Concurrently, follicular cells multiply to construct a layer of granulosa cells, and a fluid-filled antrum appears. This cavity is filled with a mix of nutritive substances. Much like the Sertoli cells in males, the granulosa cells develop gap junctions between them, thereby creating a protective barrier around the oocyte.
- Graafian follicle: Now approximately 20mm in diameter, the follicle’s antrum expands further. An external layer of thecal cells arises from the ovarian stroma. On stimulation by luteinizing hormone (LH), these cells instigate the production of estrogens, which are critical for priming the female reproductive tract for conception. The thecal cells generate androgens (primarily androstenedione) which are ultimately converted to estrogens by the granulosa cells. This conversion is catalyzed by aromatase enzyme activity and is stimulated by both FSH and LH, both of which trigger a rise in intracellular cAMP.
FSH, in tandem with estrogens, amplifies the number of LH and FSH receptors on granulosa cells, thereby heightening their sensitivity to LH/FSH.
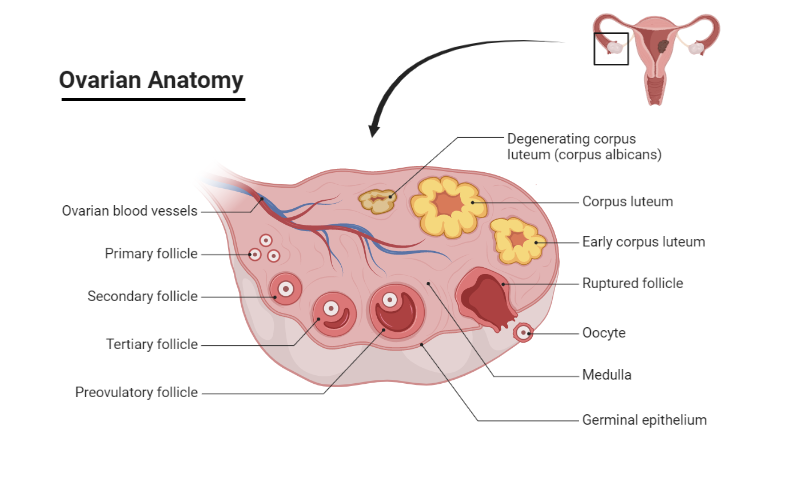
What happens after ovulation?
After ovulation, during the luteal phase, the granulosa and theca cells of the ruptured, bleeding follicle proliferate to form a yellowish corpus luteum. This structure, about 1.5 cm in diameter, persists for approximately 10 days in a non-pregnant woman. It secretes both estrogens and progesterone before eventually shrinking to form scar tissue, known as the corpus albicans.
Notably, the sharp rise in progesterone level triggers a body temperature increase of about 0.5°C, which is maintained until the end of the cycle. However, if fertilization of the ovum and implantation occurs, the corpus luteum remains active and continues steroid secretion for two to three months until this role is assumed by the placenta. By doing so, the corpus luteum ensures the maintenance of the uterine endometrium during early pregnancy.
Through this intricate process of follicular maturation and ovulation, we gain insight into the delicate orchestration of the female reproductive system—a fascinating and complex dance of hormones and cells, each playing its part in the miraculous journey toward potential life.
The Rhythms of the Uterine Cycle
The uterine cycle plays a central role in female reproduction. Each month, it coordinates a series of changes in the endometrial lining, getting the uterus ready for potential fertilization and pregnancy.
- Day 1: The cycle kicks off as the outer endometrial lining from the last cycle sheds. This results in menstrual bleeding, which is expelled through the vagina, marking the start of the menstrual phase.
- Day 5: The menstrual bleeding stops, and the cycle moves into the follicular or proliferative phase. At this stage, the developing ovarian follicles secrete estrogens, which stimulate the endometrium to grow and thicken again.
- Day 14: Around mid-cycle, a surge in the secretion of luteinizing hormone (LH) occurs. Within 24-48 hours, ovulation happens. Following ovulation, the corpus luteum, which developed from the ovulated follicle, secretes progesterone. This hormone works to prepare the estrogen-developed endometrium to become more vascular and to secrete mucus, marking the beginning of the secretory or luteal phase. This preparation is crucial for the possible implantation of a fertilized ovum.
- Day 28: If fertilization does not occur, the corpus luteum regresses, the hormonal support for the endometrium declines, and a new menstrual cycle begins. The endometrium breaks down and becomes necrotic due to the closure of the spiral arteriolar blood supply.
Concurrent with these stages, the cyclic hormonal changes also affect the consistency and pH of the cervical mucus. Around ovulation, the mucus becomes clear, watery, and stretchy, with a high pH, facilitating the passage of sperm. Post-ovulation, the mucus transforms into a viscous, cellular texture with a lower pH, creating an environment less hospitable to sperm.
In conclusion, the uterine cycle, through these meticulously orchestrated stages and accompanying physiological changes, plays a pivotal role in female fertility, ensuring that the uterus is ready for a potential pregnancy every month.
Unraveling the Hormonal Dance of the Menstrual Cycle
The menstrual cycle is an intricately coordinated interplay of hormones that leads to changes in the female reproductive system. This 28-day cycle is primarily regulated by a rise and fall in levels of certain hormones – Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), estrogen, and progesterone.
In the initial two weeks of the cycle, plasma FSH and LH levels gently rise, driving the maturation of ovarian follicles and prompting estrogen secretion. This estrogen diffuses into the bloodstream and initially curbs the secretion of FSH through a process known as negative feedback. This effect is most noticeable on FSH, although LH release is also slightly tempered.
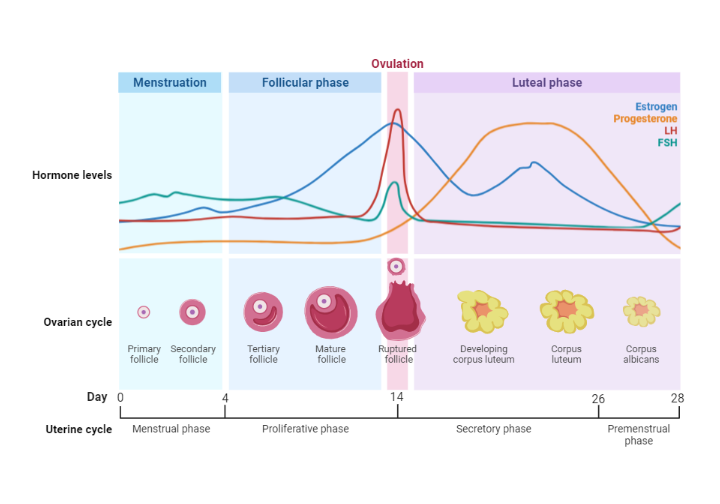
However, once estrogen levels reach a sustained high threshold, a fascinating switchover to positive feedback occurs. Instead of suppressing pituitary function, this high level of estrogen now stimulates a surge in LH that lasts for 24-48 hours. This leads to ovulation, the formation of the corpus luteum, and the secretion of progesterone. Progesterone also plays its part in the hormonal symphony by exerting a negative feedback effect on gonadotrophin secretion during the luteal phase of the cycle.
As LH levels start to decline, the corpus luteum deteriorates. With its demise, the usual negative feedback effect of estrogen on the pituitary and hypothalamus is reinstated. The ensuing drop in estrogen and progesterone levels from the corpus luteum means that the endometrium can no longer be sustained. As a result, menstruation occurs, marking the end of the cycle before the process starts anew.
An intriguing subplot in this hormonal drama is the role of inhibin. Inhibin, a peptide hormone, is produced by granulosa cells when stimulated by FSH. It plays a part in the fine-tuning of the menstrual cycle by providing some negative feedback inhibition of FSH secretion during the follicular phase. In a similar vein to its function in males, inhibin is also generated by the corpus luteum during the luteal phase.
Through this complex hormonal dance, the female body is able to prepare itself for potential fertilization every month, further highlighting the marvels of human physiology.
Decoding the Hormonal Symphony of Pregnancy
If fertilization and implantation of the ovum are successful, approximately around day 20 of the menstrual cycle, there are significant hormonal changes that ensure the continuation of the pregnancy.
One of the key players in this hormonal ensemble is human chorionic gonadotrophin (hCG), a glycoprotein hormone resembling LH in function and structure, composed of two subunits: alpha and beta. Produced by the trophoblastic cells that envelop the developing embryo, hCG makes its debut in maternal urine as early as nine days after conception, which serves as the cornerstone of most pregnancy tests.
hCG plays a critical role in the early stages of pregnancy, primarily by maintaining the corpus luteum, which in turn produces estrogen and progesterone. The consequence of this hormonal production line is the prevention of further ovulation and menstruation, ensuring the uterine lining is maintained, thereby facilitating the continuation of the pregnancy.
Around week nine of pregnancy, the placenta comes into play as a significant endocrine gland, taking over the production of estrogens (primarily oestriol, with oestradiol and oestrone) and progesterone. The placenta also continues to produce hCG, albeit in decreasing amounts. After reaching a peak in early pregnancy, hCG levels begin to decline around week 16, and the corpus luteum starts to regress.
The specific functions of hCG during fetal development or throughout the remainder of the pregnancy are not entirely clear. Nonetheless, it is thought to play a pivotal role in stimulating the Leydig cells of the male fetal testes to produce testosterone. Additionally, hCG seems to stimulate the fetal adrenal gland to produce dehydroepiandrosterone sulfate (DHEAS), an androgenic steroid precursor that the placenta utilizes to produce estrogens.
This orchestration of hormonal changes underpins the progression and maintenance of pregnancy, epitomizing the complexity and ingenuity of human reproductive physiology.
Understanding Female Sex Hormones: Spotlight on Estrogens
Diving into the world of female sex hormones, it’s impossible not to focus on estrogens, one of the key orchestrators of female reproductive physiology.
The ovary secretes the primary oestrogenic steroid, 17β-estradiol, along with oestrone (which exists in chemical equilibrium) and oestriol. Besides the ovaries, estrogens are produced by the corpus luteum and the placenta during pregnancy, with the adrenal cortex also contributing, albeit to a much smaller extent. However, only 2% of estradiol in circulation is available as a free hormone, with the rest bound to plasma albumin (60%) or sex hormone-binding globulin (SHBG) (38%). It’s worth noting that oestriol demonstrates a relatively low affinity for SHBG.
Estrogens play a pivotal role in the normal development and maintenance of the female genital tract and breasts, in addition to numerous other functions.
During puberty, estrogens orchestrate the growth of the uterus, breasts, and vagina while also controlling fat deposition and distribution in subcutaneous tissues, thereby sculpting the characteristic female figure. They trigger the adolescent “growth spurt,” similar to the role of testosterone in males, and also oversee the closure of the long bone epiphyses and the manifestation of secondary sexual characteristics. It is, however, important to note that the growth of pubic and underarm hair in females is mainly stimulated by adrenal androgen secretion.
In adulthood, estrogens continue to regulate numerous processes, including events in the menstrual cycle (notably, the growth of the endometrial lining). They play significant roles during pregnancy and lactation and contribute to the maintenance of sexual drive, often referred to as libido and female personality. Through this myriad of roles, it’s clear that estrogens represent a cornerstone of female reproductive physiology.
Unpacking Progestogens: A Closer Look at Progesterone
When we discuss female sex hormones, estrogens often take the limelight, but the lesser-discussed progestogens, particularly progesterone, are equally crucial in female reproductive physiology.
The principal progestogen produced by the ovary during the luteal phase is progesterone. It drives secretory changes in the uterine endometrium, priming it for a potential pregnancy. Alongside estrogens, progesterone also prepares the breasts for lactation by stimulating glandular development. Furthermore, progesterone modulates the cervical mucus to become thick and acidic, thereby creating a less favorable environment for sperm. Only 1-2% of progesterone in circulation is unbound, with roughly 50% being tied to albumin and the rest to transcortin, a corticosteroid-binding α2-globulin.
Progesterone is particularly important during pregnancy. It minimizes the frequency of spontaneous contractions of the myometrium, which is crucial in preventing miscarriages. Also, progesterone exerts a weak negative feedback on the anterior pituitary and hypothalamus, thereby regulating the release of LH.
Like other steroids, estrogens and progesterone exercise their effects by freely diffusing into target cells, binding with specific high-affinity intracellular estrogen or progesterone receptors. These receptors are part of a larger family of ligand-activated transcription factors (LTFs) that include receptors for thyroid hormones and glucocorticoids. Once activated, the steroid-receptor complexes undergo conformational changes, shed chaperone heat shock protein (Hsp-90), dimerize, and then bind to distinct nuclear DNA sequences. This initiates or suppresses the expression of specific genes, leading to the synthesis of new mRNA and proteins.
Interestingly, the number of estrogen and progesterone receptors in target tissues is influenced by prior estrogen stimulation. Progesterone can also reduce its own receptor numbers. Importantly, abnormalities in the expression and function of estrogen/progesterone receptors have been linked with certain health conditions, such as breast cancer, uterine fibroids, or endometriosis. Clearly, progestogens, and specifically progesterone, play an intricate and multifaceted role in female reproductive physiology.
The Two-Cell Two-Gonadotropin Model: A Snapshot of Ovarian Physiology
The two-cell two-gonadotropin model provides an elegant explanation of hormone interactions in the ovarian follicle, leading to the production of the female sex hormone estrogen. This model involves two primary cell types: the granulosa cells and the theca cells, and two gonadotropins: the follicle-stimulating hormone (FSH) and the luteinizing hormone (LH).
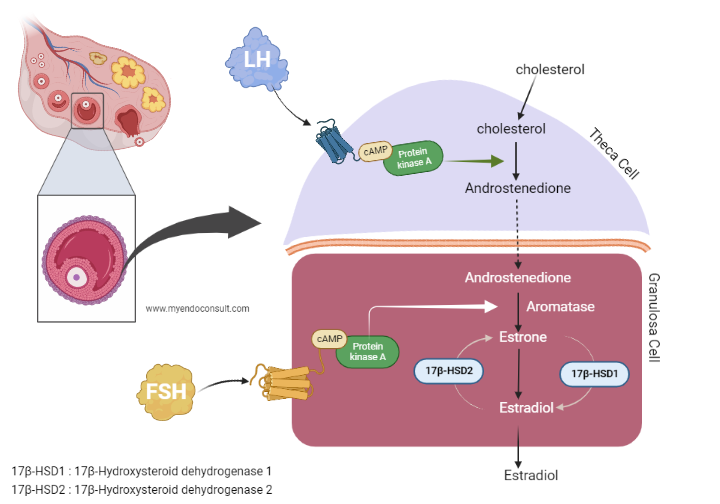
Let’s start with the theca cells. The theca cells, located outside the ovarian follicle, respond to LH stimulation by producing androgens, primarily androstenedione. However, theca cells lack the necessary enzyme, aromatase, to convert androgens to estrogen.
That’s where the granulosa cells come in. These cells, which envelop the egg within the follicle, respond to FSH stimulation and take up the androgens produced by the theca cells. Granulosa cells do possess the enzyme aromatase, which they use to convert these androgens into estrogen. Additionally, granulosa cells also produce inhibin, a hormone that provides feedback to the pituitary gland to modulate FSH secretion.
In summary, the two-cell two-gonadotropin model illustrates the elegant cooperative interplay between the granulosa and theca cells, under the influence of FSH and LH, to facilitate the production of estrogen. The feedback mechanism of inhibin adds another layer of complexity, ensuring precise control of this vital hormonal process.
Conclusion
In conclusion, the complex interplay of hormonal, cellular, and physiological processes governs female reproductive physiology. The maturation and development of ovarian follicles, orchestrated by hormonal influences such as FSH and LH, are pivotal to ovulation. Concurrently, the uterine cycle prepares the endometrium for potential implantation. Pregnancy initiates additional hormonal changes, such as the secretion of hCG, crucial in maintaining the pregnancy until the placenta assumes this role. Furthermore, female sex hormones, particularly estrogens and progesterone, have profound roles in the development and maintenance of the female reproductive system, regulation of the menstrual cycle, and fostering conditions conducive to pregnancy. The “two-cell, two-gonadotropin” model within the ovary exemplifies the synergy of various cell types and hormones in achieving the intricate process of estrogen production.
Kindly Let Us Know If This Was helpful? Thank You!


